Pharmaceutical cream with Carthamus tinctorius L. extract: formulation and evaluation
DOI:
https://doi.org/10.15587/2519-4852.2025.342475Keywords:
Carthamus tinctorius L., safflower extract, flavonoids, β-carotene, IR spectroscopy, spectrophotometry, HPLC, pharmaceutical cream, soft dosage form, formulation development, rheology, microscopyAbstract
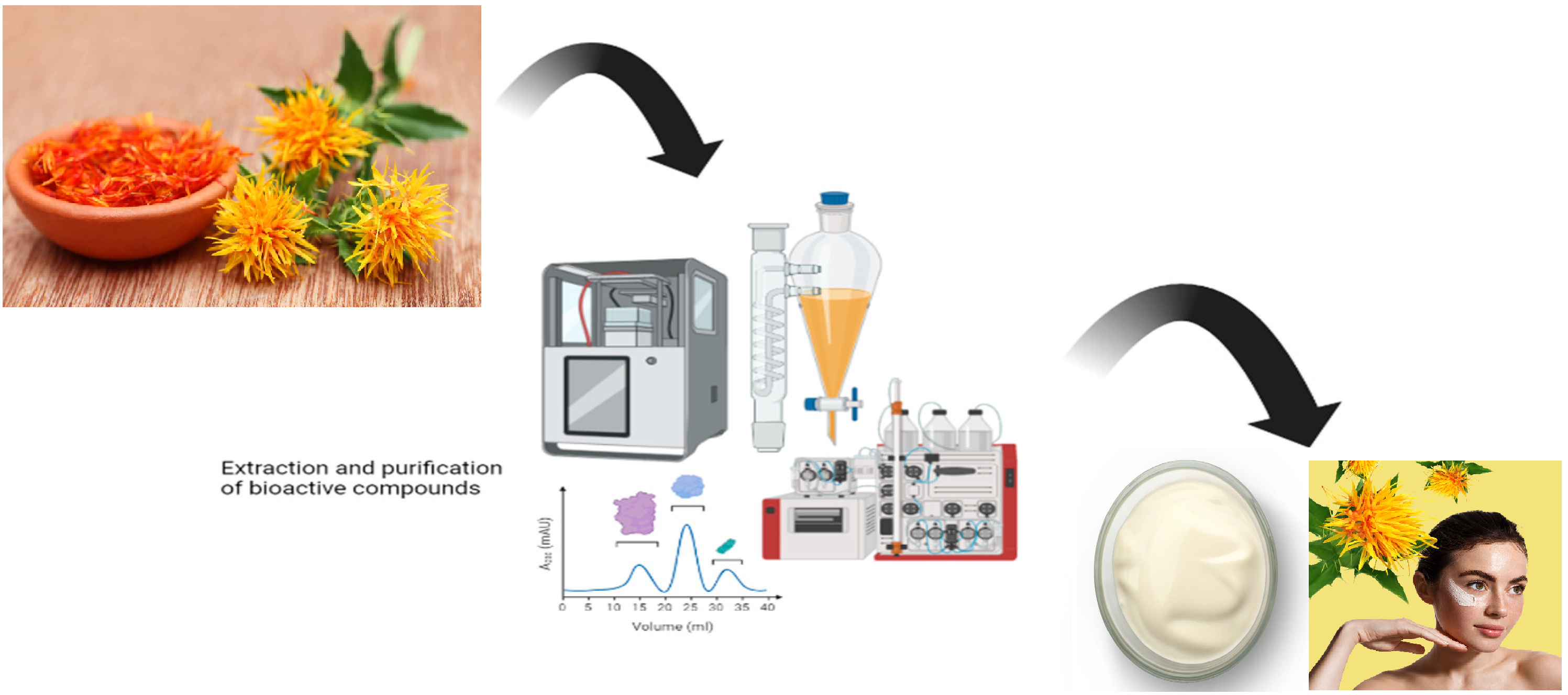
The aim. The aim of this study was to develop and evaluate a pharmaceutical cream formulation containing a liquid extract of Carthamus tinctorius L. flowers. The research focused on characterizing the extract using instrumental analytical methods (IR spectroscopy, spectrophotometry with rutin standard, HPLC, and UV spectroscopy for β-carotene) and on designing cream bases with different hydrophilic, hydrophobic, and emulsion components. The study further sought to assess the quality parameters of the developed formulations, including appearance, homogeneity, thermostability, colloidal stability, and pH, in order to identify the most stable and pharmaceutically promising compositions.
Methods. A liquid extract of Carthamus tinctorius L. flowers was used as the active pharmaceutical ingredient. Its composition and properties were analyzed using IR spectroscopy, spectrophotometry (λmax = 410 nm, rutin standard), HPLC (comparison with rutin), and UV spectroscopy for β-carotene (λmax ≈ 450 nm). Based on the extract, eight cream samples were formulated with different hydrophilic, hydrophobic, and emulsion bases. The quality of the samples was evaluated according to the following parameters: appearance, homogeneity, thermostability, colloidal stability, and pH.
Results. IR spectroscopy confirmed the presence of polyphenols, flavonoids, and organic acids. The total flavonoid content was 2.2% (calculated as rutin). HPLC analysis revealed multiple peaks, including rutin (Rt = 18.17 min), coinciding with the standard (Rt = 18.20 min). The UV spectrum showed a high level of β-carotene (A = 4.35 compared to A = 0.60 in the standard). Out of the 8 cream samples, only formulations No. 3, No. 5, and No. 7 demonstrated stability.
Discussion. Instrumental analysis confirmed the presence of a complex of biologically active substances in the extract, ensuring its anti-inflammatory and antioxidant properties. The high content of flavonoids and β-carotene substantiates the therapeutic potential of the product. Selection of the cream base showed that a rational combination of hydrophilic and hydrophobic components ensures stability and preservation of active ingredients.
Conclusions. A therapeutic-cosmetic cream with a liquid extract of Carthamus tinctorius L. has been developed. Out of 8 tested formulations, samples No. 3, No. 5, and No. 7 demonstrated the best quality parameters and are recommended for further investigation. The obtained data confirm the prospects of using safflower extract in the creation of modern phytopharmaceuticals with pronounced anti-inflammatory and antioxidant activity
References
- Sahu, T., Patel, T., Sahu, S., Gidwani, B. (2016). Skin cream as topical drug delivery system: a review. Journal of Pharmaceutical and Biological Sciences, 4 (5), 149. Available at: https://www.researchgate.net/publication/321825248_Skin_Cream_as_Topical_Drug_Delivery_System_A_Review
- Asgarpanah, J., Kazemivash, N. (2013). Phytochemistry, pharmacology and medicinal properties of Carthamus tinctorius L. Chinese Journal of Integrative Medicine, 19 (2), 153–159. https://doi.org/10.1007/s11655-013-1354-5
- Delshad, E., Yousefi, M., Sasannezhad, P., Rakhshandeh, H., Ayati, Z. (2018). Medical uses of Carthamus tinctorius L. (Safflower): a comprehensive review from Traditional Medicine to Modern Medicine. Electronic Physician, 10 (4), 6672–6681. https://doi.org/10.19082/6672
- Lee, J. Y., Chang, E. J., Kim, H. J., Park, J. H., Choi, S. W. (2002). Antioxidative flavonoids from leaves of Carthamus tinctorius. Archives of Pharmacal Research, 25 (3), 313–319. https://doi.org/10.1007/bf02976632
- Ekin, Z. (2005). Resurgence of Safflower (Carthamus tinctorius L.) Utilization: A Global View. Journal of Agronomy, 4 (2), 83–87. https://doi.org/10.3923/ja.2005.83.87
- Emongor, V. (2010). Safflower (Carthamus tinctorius L.) the Underutilized and Neglected Crop: A Review. Asian Journal of Plant Sciences, 9 (6), 299–306. https://doi.org/10.3923/ajps.2010.299.306
- Bakhtiyor kizi, S. I., Shakhsaidovich, S. S., Ravshan kizi, K. A., Gulrano, A., Khalimovna, R. N., Bakhtiyarovna, T. D. (2025). Morphological and size characterization of zinc oxide nanoparticles and evaluation of their cytotoxicity on the MCF-7 cell line. ScienceRise: Pharmaceutical Science, 4 (56), 88–96. https://doi.org/10.15587/2519-4852.2025.338297
- Akhmadova, G., Mirrakhimova, T., Ismoilova, G. (2024). High-quality analysis of dry extract of prickly artichoke raw material (Cynara Scolymus L.) cultivated in Uzbekistan. ScienceRise: Pharmaceutical Science, 4 (50), 60–66. https://doi.org/10.15587/2519-4852.2024.310826
- Olimov, K., Mirrakhimova, T., Akhmadova, G. (2025). Polysaccharide profile, acute toxicity and bile secretion effects of the choleretic herbal preparation “Safroart herbal tea.” ScienceRise: Pharmaceutical Science, 2 (54), 59–68. https://doi.org/10.15587/2519-4852.2025.327605
- Trommer, H., Neubert, R. H. H. (2006). Overcoming the Stratum Corneum: The Modulation of Skin Penetration. Skin Pharmacology and Physiology, 19 (2), 106–121. Portico. https://doi.org/10.1159/000091978
- Pinsky, M. A. (2017). Efficacy and safety of an anti-aging technology for the treatment of facial wrinkles and skin moisturization. The Journal of clinical and aesthetic dermatology, 10 (12), 27–35. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5774901/
- Uktamov, B., Rizayeva, N. M., Mirzakamalova, D. S., Sharipova, I. Sh. (2021). Development of the Composition, Technology and Study of the Effectiveness of Drops for Oral Administration "Ascorbicdrop". Journal of Pharmaceutical Research International, 33 (56), 310–316. https://doi.org/10.9734/jpri/2021/v33i56B33957
- Chang, Y., Shi, X., He, F., Wu, T., Jiang, L., Normakhamatov, N. et al. (2022). Valorization of Food Processing Waste to Produce Valuable Polyphenolics. Journal of Agricultural and Food Chemistry, 70 (29), 8855–8870. https://doi.org/10.1021/acs.jafc.2c02655
- El Sohafy, S. M., Nassra, R. A., D’Urso, G., Piacente, S., Sallam, S. M. (2020). Chemical profiling and biological screening with potential anti-inflammatory activity of Callisia fragrans grown in Egypt. Natural Product Research, 35 (23), 5521–5524. https://doi.org/10.1080/14786419.2020.1791113
- Boligon, A. A., Athayde, M. L. (2014). Importance of HPLC in analysis of plant extracts. Austin Chromatography, 1 (3), 2. Available at: https://austinpublishinggroup.com/chromatography/fulltext/chromatography-v1-id1011.php
- Proestos, C., Chorianopoulos, N., Nychas, G.-J. E., Komaitis, M. (2005). RP-HPLC Analysis of the Phenolic Compounds of Plant Extracts. Investigation of Their Antioxidant Capacity and Antimicrobial Activity. Journal of Agricultural and Food Chemistry, 53 (4), 1190–1195. https://doi.org/10.1021/jf040083t
- Engida, A. M., Kasim, N. S., Tsigie, Y. A., Ismadji, S., Huynh, L. H., Ju, Y.-H. (2013). Extraction, identification and quantitative HPLC analysis of flavonoids from sarang semut (Myrmecodia pendan). Industrial Crops and Products, 41, 392–396. https://doi.org/10.1016/j.indcrop.2012.04.043
- Mantzouris, D., Karapanagiotis, I., Panayiotou, C. (2014). Comparison of extraction methods for the analysis of Indigofera tinctoria and Carthamus tinctorius in textiles by high performance liquid chromatography. Microchemical Journal, 115, 78–86. https://doi.org/10.1016/j.microc.2014.02.010
- Johansen, K. T., Wubshet, S. G., Nyberg, N. T., Jaroszewski, J. W. (2011). From Retrospective Assessment to Prospective Decisions in Natural Product Isolation: HPLC-SPE-NMR Analysis of Carthamus oxyacantha. Journal of Natural Products, 74 (11), 2454–2461. https://doi.org/10.1021/np200780m
- Wang, Y., Chen, P., Tang, C., Wang, Y., Li, Y., Zhang, H. (2014). Antinociceptive and anti-inflammatory activities of extract and two isolated flavonoids of Carthamus tinctorius L. Journal of Ethnopharmacology, 151 (2), 944–950. https://doi.org/10.1016/j.jep.2013.12.003
- Meinhardt-Wollweber, M., Suhr, C., Kniggendorf, A.-K., Roth, B. (2018). Absorption and resonance Raman characteristics of β-carotene in water-ethanol mixtures, emulsion and hydrogel. AIP Advances, 8 (5). https://doi.org/10.1063/1.5025788
- Hagos, M., Redi-Abshiro, M., Chandravanshi, B. S., Yaya, E. E. (2022). Development of Analytical Methods for Determination of β-Carotene in Pumpkin (Cucurbita maxima) Flesh, Peel, and Seed Powder Samples. International Journal of Analytical Chemistry, 2022, 1–11. https://doi.org/10.1155/2022/9363692
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2025 Nilufar Rizaeva, Gulrano Akhmadova, Munozhat Akhmedova, Dilnoza Bakhriddinova, Nozima Aripova, Irodakhon Sharipova, Nigora Azlarova, Umida Anarmetova

This work is licensed under a Creative Commons Attribution 4.0 International License.
Our journal abides by the Creative Commons CC BY copyright rights and permissions for open access journals.







