Синтез, оцінка протиракових властивостей та in silico дослідження 2-хлоро- та 2,2-дихлороацетамідовмісних тіазольних похідних
DOI:
https://doi.org/10.15587/2519-4852.2025.323594Ключові слова:
хлороацетаміди, дихлороацетаміди, амінотіазоли, протиракова активність, квантові розрахунки, молекулярний докінг, глутатіон, інгібування GSTАнотація
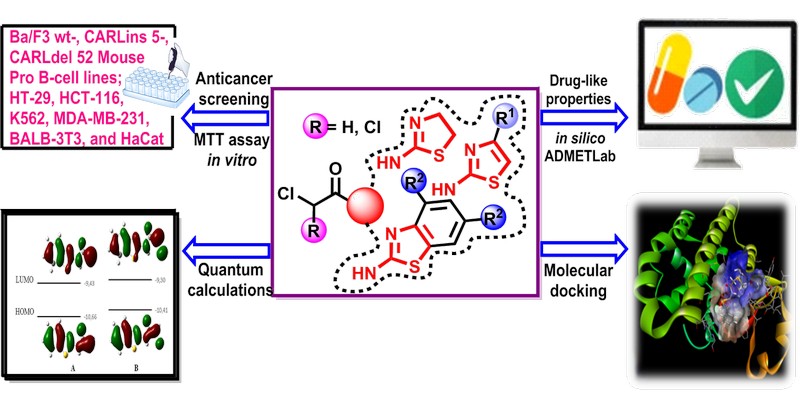
Мета. Метою дослідження був синтез та оцінка протиракової активності серії 2-хлоро- та 2,2-дихлороацетамідовмісних тіазольних похідних. Особлива увага приділялася їх цитотоксичним ефектам, хімічним властивостям та можливому механізму дії, з акцентом на інгібування глутатіон-S-трансферази (GST), як потенційного шляху протиракової активності.
Матеріали та методи. Сполуки були синтезовані в реакціях ацилювання та охарактеризовані методами спектроскопії 1Н та 13С ЯМР, а також хромато-мас-спектрометрії. Цитотоксичність оцінювали за допомогою аналізу МТТ на ракових і псевдо-нормальних клітинних лініях. Квантово-хімічні розрахунки проводилися за допомогою DFT, тоді як інгібування GST вивчали методом молекулярного докінгу.
Результати. Серед синтезованих похідних 2-хлороацетаміди виявили значну цитотоксичну активність щодо клітин гострого Т-клітинного лейкозу людини (Jurkat) і потрійного негативного раку молочної залози (MDA-MB-231), а також клітин Ba/F3 з мутаціями кальретикуліну. У той же час, 2,2-дихлороацетаміди показали незначну активність у всіх перевірених клітинних лініях. Квантово-хімічний аналіз показав, що структурні та електронні відмінності між цими двома класами сполук, ймовірно, впливають на їх біоактивність. Дослідження методом молекулярного докінгу виявили більш високу афінність зв’язування кон’югатів глутатіон-2-хлороацетамідів з GST порівняно з еталонним комплексом глутатіон-етакринова кислота, що свідчить про інгібування GST як потенційний механізм, що лежить в основі їх протиракової дії.
Висновки. Синтезовані 2-хлороацетаміди демонструють певний потенціал як потенційні протипухлинні засоби, ймовірно, завдяки їх здатності утворювати інгібіторні кон’югати з глутатіоном, тим самим впливаючи на активність GST. Ці висновки підкреслюють важливість подальших досліджень для оптимізації цих сполук для можливого терапевтичного використання
Спонсор дослідження
- This research was funded by the National Research Foundation of Ukraine Grant № 2023.05/0021 and Grant № 2023.03/0104
Посилання
- Bedard, P. L., Hyman, D. M., Davids, M. S., Siu, L. L. (2020). Small molecules, big impact: 20 years of targeted therapy in oncology. The Lancet, 395 (10229), 1078–1088. https://doi.org/10.1016/s0140-6736(20)30164-1
- Zhong, L., Li, Y., Xiong, L., Wang, W., Wu, M., Yuan, T. et al. (2021). Small molecules in targeted cancer therapy: advances, challenges, and future perspectives. Signal Transduction and Targeted Therapy, 6 (1). https://doi.org/10.1038/s41392-021-00572-w
- Olgen, S. (2018). Overview on Anticancer Drug Design and Development. Current Medicinal Chemistry, 25 (15), 1704–1719. https://doi.org/10.2174/0929867325666171129215610
- Steinbrueck, A., Sedgwick, A. C., Brewster, J. T., Yan, K.-C., Shang, Y., Knoll, D. M. et al. (2020). Transition metal chelators, pro-chelators, and ionophores as small molecule cancer chemotherapeutic agents. Chemical Society Reviews, 49(12), 3726–3747. https://doi.org/10.1039/c9cs00373h
- Zhang, J., Duan, D., Song, Z., Liu, T., Hou, Y., Fang, J. (2020). Small molecules regulating reactive oxygen species homeostasis for cancer therapy. Medicinal Research Reviews, 41 (1), 342–394. https://doi.org/10.1002/med.21734
- Hassan, G. S., El-Messery, S. M., Al-Omary, F. A. M., El-Subbagh, H. I. (2012). Substituted thiazoles VII. Synthesis and antitumor activity of certain 2-(substituted amino)-4-phenyl-1,3-thiazole analogs. Bioorganic & Medicinal Chemistry Letters, 22 (20), 6318–6323. https://doi.org/10.1016/j.bmcl.2012.08.095
- Padhariya, K. N., Athavale, M., Srivastava, S., Kharkar, P. S. (2019). Substituted chloroacetamides as potential cancer stem cell inhibitors: Synthesis and biological evaluation. Drug Development Research, 81 (3), 356–365. https://doi.org/10.1002/ddr.21628
- Demirci, S., Uslu, H., Mermer, A., Yesilay, G., Betul Aydin, Z. (2024). Synthesis and Characterization of Propofol‐Like Compounds, Anticancer Activities and Investigation of Their Anesthetic Effects by Molecular Docking. ChemistrySelect, 9 (17). https://doi.org/10.1002/slct.202400431
- Lesyk, R., Vladzimirska, O., Zimenkovsky, B., Golota, S., Nektgayev, I., Cherpak, O. et al. (2002). Synthesis and antiinflammatory activity of novel 3-(2,3-dimethyl-1-phenyl-4-pyrazolon-5-yl)-4-thiazolidones. Bollettino Chimico Farmaceutico, 141 (3), 197–201.
- Havryshchuk, L., Horishny, V., Rushchak, N., Lesyk, R. (2024). Dichloroacetic acid derivatives as potential anti-tumor and anti-inflammatory agents. ScienceRise: Pharmaceutical Science, 1 (47), 60–78. https://doi.org/10.15587/2519-4852.2024.299229
- Fumarola, C., Bozza, N., Castelli, R., Ferlenghi, F., Marseglia, G., Lodola, A. et al. (2019). Expanding the Arsenal of FGFR Inhibitors: A Novel Chloroacetamide Derivative as a New Irreversible Agent With Anti-proliferative Activity Against FGFR1-Amplified Lung Cancer Cell Lines. Frontiers in Oncology, 9. https://doi.org/10.3389/fonc.2019.00179
- Bogdanović, A., Marinković, A., Stanojković, T., Grozdanić, N., Janakiev, T., Cvijetić, I., Petrović, S. (2025). Synthesis, antimicrobial, anticancer activity, 3D QSAR, ADMET properties, and in silico target fishing of novel N,N-disubstituted chloroacetamides. Journal of Molecular Structure, 1321, 140075. https://doi.org/10.1016/j.molstruc.2024.140075
- Müller, M. P., Jeganathan, S., Heidrich, A., Campos, J., Goody, R. S. (2017). Nucleotide based covalent inhibitors of KRas can only be efficient in vivo if they bind reversibly with GTP-like affinity. Scientific Reports, 7 (1). https://doi.org/10.1038/s41598-017-03973-6
- Xiong, Y., Lu, J., Hunter, J., Li, L., Scott, D., Choi, H. G. et al. (2016). Covalent Guanosine Mimetic Inhibitors of G12C KRAS. ACS Medicinal Chemistry Letters, 8 (1), 61–66. https://doi.org/10.1021/acsmedchemlett.6b00373
- Hossain, M., Roayapalley, P. K., Sakagami, H., Satoh, K., Bandow, K., Das, U., Dimmock, J. R. (2022). Dichloroacetyl Amides of 3,5-Bis(benzylidene)-4-piperidones Displaying Greater Toxicity to Neoplasms than to Non-Malignant Cells. Medicines, 9 (6), 35. https://doi.org/10.3390/medicines9060035
- Fereidoonnezhad, M., Tabaei, S. M. H., Sakhteman, A., Seradj, H., Faghih, Z., Faghih, Z. et al. (2020). Design, synthesis, molecular docking, biological evaluations and QSAR studies of novel dichloroacetate analogues as anticancer agent. Journal of Molecular Structure, 1221, 128689. https://doi.org/10.1016/j.molstruc.2020.128689
- Zare, S., Ramezani, Z., Ghadiri, A. A., Fereidoonnezhad, M. (2023). Synthesis of N‐(2‐(tert‐Butylamino)‐2‐oxoethyl)‐2,2‐dichloro‐N‐aryl(alkyl)acetamides as Anticancer Agents: Molecular Modeling and Biological Evaluations. ChemistrySelect, 8 (1). https://doi.org/10.1002/slct.202203931
- Li, T., Yang, Y., Cheng, C., Tiwari, A. K., Sodani, K., Zhao, Y. et al. (2012). Design, synthesis and biological evaluation of N-arylphenyl-2,2-dichloroacetamide analogues as anti-cancer agents. Bioorganic & Medicinal Chemistry Letters, 22 (23), 7268–7271. https://doi.org/10.1016/j.bmcl.2012.07.057
- Zhang, S.-L., Zhang, W., Xiao, Q., Yang, Z., Hu, X., Wei, Z., Tam, K. Y. (2016). Development of dichloroacetamide pyrimidines as pyruvate dehydrogenase kinase inhibitors to reduce cancer cell growth: synthesis and biological evaluation. RSC Advances, 6 (82), 78762–78767. https://doi.org/10.1039/c6ra14060b
- Trapella, C., Voltan, R., Melloni, E., Tisato, V., Celeghini, C., Bianco, S. et al. (2015). Design, Synthesis, and Biological Characterization of Novel Mitochondria Targeted Dichloroacetate-Loaded Compounds with Antileukemic Activity. Journal of Medicinal Chemistry, 59 (1), 147–156. https://doi.org/10.1021/acs.jmedchem.5b01165
- Zhang, S.-L., Hu, X., Zhang, W., Yao, H., Tam, K. Y. (2015). Development of pyruvate dehydrogenase kinase inhibitors in medicinal chemistry with particular emphasis as anticancer agents. Drug Discovery Today, 20 (9), 1112–1119. https://doi.org/10.1016/j.drudis.2015.03.012
- Hossain, M., Roth, S., Dimmock, J. R., Das, U. (2022). Cytotoxic derivatives of dichloroacetic acid and some metal complexes. Archiv Der Pharmazie, 355 (11). https://doi.org/10.1002/ardp.202200236
- Yang, Y., Shang, P., Cheng, C., Wang, D., Yang, P., Zhang, F. et al. (2010). Novel N-phenyl dichloroacetamide derivatives as anticancer reagents: Design, synthesis and biological evaluation. European Journal of Medicinal Chemistry, 45 (9), 4300–4306. https://doi.org/10.1016/j.ejmech.2010.06.032
- Abdel‐Latif, E., Fahad, M. M., El‐Demerdash, A., Ismail, M. A. (2020). Synthesis and biological evaluation of some heterocyclic scaffolds based on the multifunctional N‐(4‐acetylphenyl)‐2‐chloroacetamide. Journal of Heterocyclic Chemistry, 57 (8), 3071–3081. https://doi.org/10.1002/jhet.4012
- Zimenkovsky, B., Lesyk, R., Vladzimirska, O., Nektegayev, I., Golota, S., Chorniy, I. (1999). The structure – anti-inflammatory activity relationship among thiazolidones: Conclusion from scientific programme. Journal of Pharmacy and Pharmacology, 51, 264.
- Abdel-Latif, E., Fahad, M. M., Ismail, M. A. (2019). Synthesis ofN-aryl 2-chloroacetamides and their chemical reactivity towards various types of nucleophiles. Synthetic Communications, 50 (3), 289–314. https://doi.org/10.1080/00397911.2019.1692225
- Huang, F., Han, X., Xiao, X., Zhou, J. (2022). Covalent Warheads Targeting Cysteine Residue: The Promising Approach in Drug Development. Molecules, 27 (22), 7728. https://doi.org/10.3390/molecules27227728
- Bonnet, S., Archer, S. L., Allalunis-Turner, J., Haromy, A., Beaulieu, C., Thompson, R. et al. (2007). A Mitochondria-K+ Channel Axis Is Suppressed in Cancer and Its Normalization Promotes Apoptosis and Inhibits Cancer Growth. Cancer Cell, 11 (1), 37–51. https://doi.org/10.1016/j.ccr.2006.10.020
- Koltai, T., Fliegel, L. (2024). Dichloroacetate for Cancer Treatment: Some Facts and Many Doubts. Pharmaceuticals, 17 (6), 744. https://doi.org/10.3390/ph17060744
- Alizadeh, S. R., Hashemi, S. M. (2021). Development and therapeutic potential of 2-aminothiazole derivatives in anticancer drug discovery. Medicinal Chemistry Research, 30 (4), 771–806. https://doi.org/10.1007/s00044-020-02686-2
- Wan, Y., Long, J., Gao, H., Tang, Z. (2021). 2-Aminothiazole: A privileged scaffold for the discovery of anti-cancer agents. European Journal of Medicinal Chemistry, 210, 112953. https://doi.org/10.1016/j.ejmech.2020.112953
- Mishchenko, M., Shtrygol’, S., Lozynskyi, A., Khomyak, S., Novikov, V., Karpenko, O. et al. (2021). Evaluation of Anticonvulsant Activity of Dual COX-2/5-LOX Inhibitor Darbufelon and Its Novel Analogues. Scientia Pharmaceutica, 89 (2), 22. https://doi.org/10.3390/scipharm89020022
- Geronikaki, A., Theophilidis, G. (1992). Synthesis of 2-(aminoacetylamino)thiazole derivatives and comparison of their local anaesthetic activity by the method of action potential. European Journal of Medicinal Chemistry, 27 (7), 709–716. https://doi.org/10.1016/0223-5234(92)90091-e
- Ivasechko, I., Yushyn, I., Roszczenko, P., Senkiv, J., Finiuk, N., Lesyk, D. et al. (2022). Development of Novel Pyridine-Thiazole Hybrid Molecules as Potential Anticancer Agents. Molecules, 27 (19), 6219. https://doi.org/10.3390/molecules27196219
- Frisch, M., Trucks, G., Schlegel, H., Scuseria, G., Robb, M., Cheeseman, J. et al. (2016). Gaussian 09, Revision A.02. Wallingford: Gaussian Inc.
- Dennington, R., Keith, T., Millam, J. (2016). GaussView 5.0.8. Shawnee Mission: Semichem Inc.
- Halgren, T. A. (1996). Merck molecular force field. I. Basis, form, scope, parameterization, and performance of MMFF94. Journal of Computational Chemistry, 17 (5-6), 490–519. https://doi.org/10.1002/(sici)1096-987x(199604)17:5/6<490::aid-jcc1>3.0.co;2-p
- Hanwell, M. D., Curtis, D. E., Lonie, D. C., Vandermeersch, T., Zurek, E., Hutchison, G. R. (2012). Avogadro: an advanced semantic chemical editor, visualization, and analysis platform. Journal of Cheminformatics, 4 (1). https://doi.org/10.1186/1758-2946-4-17
- Oakley, A. J., Lo Bello, M., Mazzetti, A. P., Federici, G., Parker, M. W. (1997). The glutathione conjugate of ethacrynic acid can bind to human pi class glutathione transferase P1‐1 in two different modes. FEBS Letters, 419 (1), 32–36. https://doi.org/10.1016/s0014-5793(97)01424-5
- Kramer, B., Rarey, M., Lengauer, T. (1999). Evaluation of the FLEXX incremental construction algorithm for protein-ligand docking. Proteins: Structure, Function, and Genetics, 37 (2), 228–241. https://doi.org/10.1002/(sici)1097-0134(19991101)37:2<228::aid-prot8>3.0.co;2-8
- Yusuf, D., Davis, A. M., Kleywegt, G. J., Schmitt, S. (2008). An Alternative Method for the Evaluation of Docking Performance: RSR vs RMSD. Journal of Chemical Information and Modeling, 48 (7), 1411–1422. https://doi.org/10.1021/ci800084x
- Yushyn, I., Holota, S., Lesyk, R. (2022). 2,2-Dichloro-N-[5-[2-[3-(4-methoxyphenyl)-5-phenyl-3,4-dihydro-2H-pyrazol-2-yl]-2-oxoethyl]sulfanyl-1,3,4-thiadiazol-2-yl]acetamide. Molbank, 2022(1), M1328. https://doi.org/10.3390/m1328
- ADMETlab 3.0. Scbdd.com. Available at: https://admetlab3.scbdd.com/server/evaluation
- Ree, N., Göller, A. H., Jensen, J. H. (2024). Automated quantum chemistry for estimating nucleophilicity and electrophilicity with applications to retrosynthesis and covalent inhibitors. Digital Discovery, 3 (2), 347–354. https://doi.org/10.1039/d3dd00224a
- Yamane, D., Tetsukawa, R., Zenmyo, N., Tabata, K., Yoshida, Y., Matsunaga, N. et al. (2023). Expanding the Chemistry of Dihaloacetamides as Tunable Electrophiles for Reversible Covalent Targeting of Cysteines. Journal of Medicinal Chemistry, 66 (13), 9130–9146. https://doi.org/10.1021/acs.jmedchem.3c00737
- Coleman, S., Linderman, R., Hodgson, E., Rose, R. L. (2000). Comparative metabolism of chloroacetamide herbicides and selected metabolites in human and rat liver microsomes. Environmental Health Perspectives, 108 (12), 1151–1157. https://doi.org/10.1289/ehp.001081151
- Bernasinska, J., Duchnowicz, P., Koter-Michalak, M., Koceva-Chyla, A. (2013). Effect of safeners on damage of human erythrocytes treated with chloroacetamide herbicides. Environmental Toxicology and Pharmacology, 36 (2), 368–377. https://doi.org/10.1016/j.etap.2013.04.010
- Jablonkai, I., Dutka, F. (1992). Preparative-scale synthesis and physicochemical properties of cysteine and glutathione conjugates of chloroacetamides. Journal of Agricultural and Food Chemistry, 40 (3), 506–508. https://doi.org/10.1021/jf00015a029
- Ma, X., Zhang, Y., Guan, M., Zhang, W., Tian, H., Jiang, C. et al. (2021). Genotoxicity of chloroacetamide herbicides and their metabolites in vitro and in vivo. International Journal of Molecular Medicine, 47 (6). https://doi.org/10.3892/ijmm.2021.4936
- Ploemen, J. H. T. M., Ommen, B. V., Bogaards, J. J. P., Van Bladeren, P. J. (1993). Ethacrynic acid and its glutathione conjugate as inhibitors of glutathioneS-transferases. Xenobiotica, 23 (8), 913–923. https://doi.org/10.3109/00498259309059418
- Burgess, E. R., Mishra, S., Yan, X., Guo, Z., Geden, C. J., Miller, J. S., Scharf, M. E. (2024). Differential interactions of ethacrynic acid and diethyl maleate with glutathione S-transferases and their glutathione co-factor in the house fly. Pesticide Biochemistry and Physiology, 205, 106170. https://doi.org/10.1016/j.pestbp.2024.106170
- Emre Erat, Y., Rümeysa Erat, A., Erat, M. (2023). Inhibitory Effect of Ascorbic Acid on Glutathione S-transferase from Human Erythrocytes. Current Enzyme Inhibition, 19 (3), 188–194. https://doi.org/10.2174/1573408019666230530095315
- Hellou, J., Ross, N. W., Moon, T. W. (2012). Glutathione, glutathione S-transferase, and glutathione conjugates, complementary markers of oxidative stress in aquatic biota. Environmental Science and Pollution Research, 19 (6), 2007–2023. https://doi.org/10.1007/s11356-012-0909-x
- Tew, K. D. (2016). Glutathione-Associated Enzymes In Anticancer Drug Resistance. Cancer Research, 76 (1), 7–9. https://doi.org/10.1158/0008-5472.can-15-3143
- Mahajan, S., Atkins, W. M. (2005). The chemistry and biology of inhibitors and pro-drugs targeted to glutathione S-transferases. Cellular and Molecular Life Sciences, 62 (11), 1221–1233. https://doi.org/10.1007/s00018-005-4524-6
- Townsend, D. M., Tew, K. D. (2003). The role of glutathione-S-transferase in anti-cancer drug resistance. Oncogene, 22 (47), 7369–7375. https://doi.org/10.1038/sj.onc.1206940
- Gülçin, İ., Scozzafava, A., Supuran, C. T., Akıncıoğlu, H., Koksal, Z., Turkan, F., Alwasel, S. (2015). The effect of caffeic acid phenethyl ester (CAPE) on metabolic enzymes including acetylcholinesterase, butyrylcholinesterase, glutathione S-transferase, lactoperoxidase, and carbonic anhydrase isoenzymes I, II, IX, and XII. Journal of Enzyme Inhibition and Medicinal Chemistry, 31 (6), 1095–1101. https://doi.org/10.3109/14756366.2015.1094470
- Ozgencli, I., Kilic, D., Guller, U., Ciftci, M., Kufrevioglu, O. I., Budak, H. (2019). A Comparison of the Inhibitory Effects of Anti-Cancer Drugs on Thioredoxin Reductase and Glutathione S-Transferase in Rat Liver. Anti-Cancer Agents in Medicinal Chemistry, 18 (14), 2053–2061. https://doi.org/10.2174/1871520618666180910093335
##submission.downloads##
Опубліковано
Як цитувати
Номер
Розділ
Ліцензія
Авторське право (c) 2025 Liubomyr Havryshchuk, Volodymyr Horishny, Iryna Ivasechko, Yuliia Kozak, Dmytro Melnyk, Dmytro Khylyuk, Myroslava Kusiy, Victoria Serhiyenko, Nataliya Finiuk, Rostyslav Stoika, Serhii Holota, Roman Lesyk

Ця робота ліцензується відповідно до Creative Commons Attribution 4.0 International License.
Наше видання використовує положення про авторські права Creative Commons CC BY для журналів відкритого доступу.







