Застосування алгоритмів кластеризації та фармакофорного скринінгу для ідентифікації похідних тіазолідинону та піразоліну з подвійною протипаразитарною та протираковою активністю
DOI:
https://doi.org/10.15587/2519-4852.2025.328968Ключові слова:
машинне навчання, тіазолідинон, піразолін, антитрипаносомна активність, протипухлинна активність, фармакофорний дизайнАнотація
Тіазолідинони та споріднені гетероцикли, що проявляють антимікробну, протипаразитарну, протидіабетичну та протизапальну активність, є привілейованими скафолдами для розробки нових лікоподібних молекул. Гібриди на основі 4-тіазолідинонів з фрагментами алканкарбонової кислоти, піразоліном, феніліндолом або імідазотіадіазолу широко вивчалися як потенційні протипаразитарні засоби. Поряд з численними дослідженнями, які довели їхній високий протипухлинний потенціал, цей клас сполук є привабливим для перспективної стратегії перепрофілювання протипаразитарних препаратів для терапії раку.
Мета дослідження. Метою дослідження був пошук кореляції між протилейкемічними та протипаразитарними властивостями низки різних похідних тіазолідинону та піразоліну.
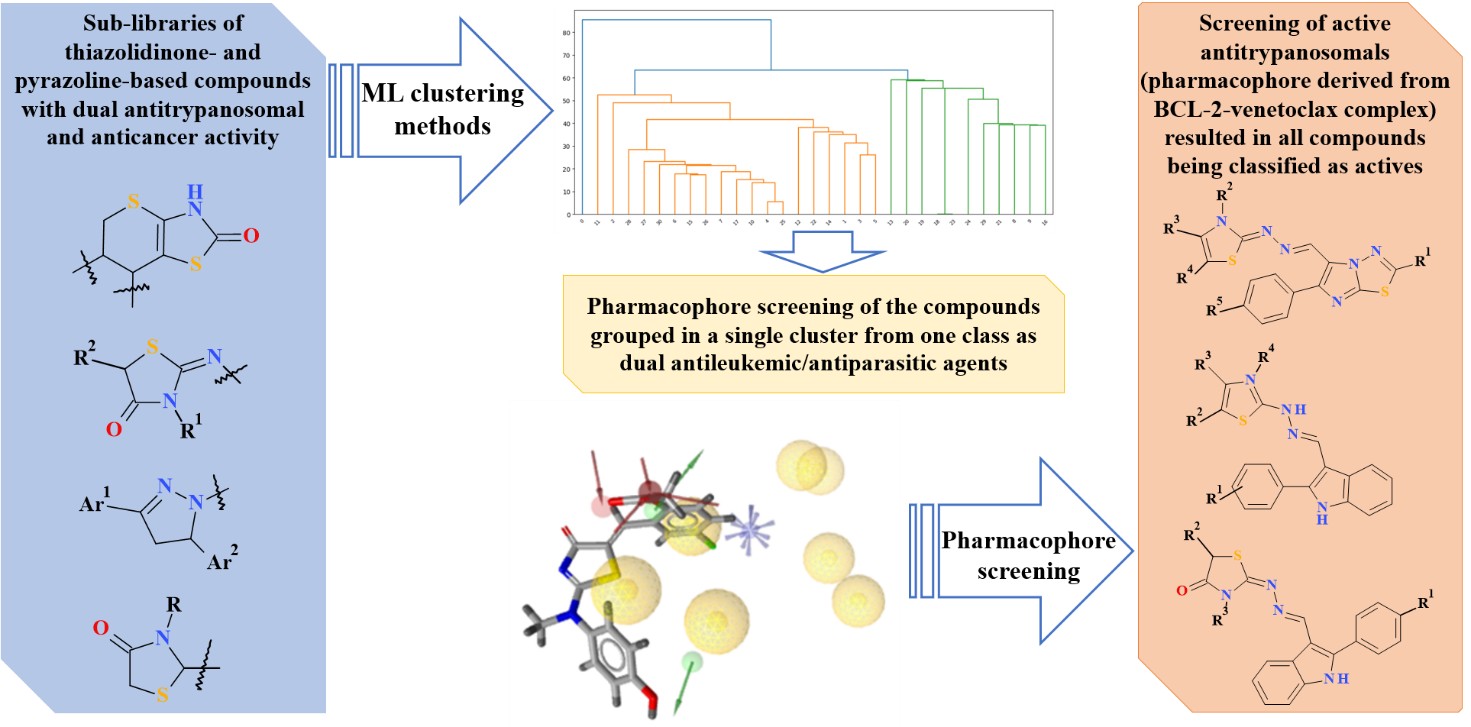
Матеріали та методи. Протиракову активність вибірки із 31 сполуки щодо п’яти лейкемічних клітинних ліній вивчали в одній концентрації (10−5M). Дані про антитрипаносомну активність отримували за умов однакових протоколів біологічних досліджень щодо Trypanosoma brucei brucei (Tbb). Алгоритми кластеризації проводили у мові програмування Python з використанням бібліотек NumPy, Pandas, Scikit-learn, Matplotlib та Plotly. Для розробки 3D-фармакофору використовували програмне забезпечення LigandScout 4.4.
Результати. Сполуки з антитрипаносомною активністю були розділені на 3 класи відповідно до значень IC50, розрахованих в тесті інгібування росту Tbb. Для проведення машинного навчання було використано відсоток росту клітин досліджуваних сполук в in vitro тесті на п'яти лейкемічних клітинних лініях. Застосовуючи кластеризацію обома методами K-середніх та Агломеративним ієрархічним алгоритмом, сполуки з класу 1 були згруповані в один кластер. Скринінг фармакофорів з використанням об'єднаного фармакофору, отриманого з комплексів BCL-2-венетоклакс, показав хороші показники відповідності фармакофорів для сполук, відібраних в один кластер обома алгоритмами. При застосуванні тієї ж фармакофорної моделі до вибірки гібридних молекул тіазолідинону/тіазол-індол/імідазотіадіазолу з високою антитрипаносомною активністю in vitro, дані сполуки були класифіковані як активні.
Висновки. Результати дослідження свідчать про те, що похідні тіазолідину та споріднені сполуки проявляють подвійні протипаразитарні та протипухлинні властивості, що може допомогти у пошуку їхнього антипроліферативного механізму дії в паразитарних та пухлинних клітинах
Посилання
- Kaminskyy, D., Kryshchyshyn, A., Lesyk, R. (2017). 5-Ene-4-thiazolidinones – An efficient tool in medicinal chemistry. European Journal of Medicinal Chemistry, 140, 542–594. https://doi.org/10.1016/j.ejmech.2017.09.031
- Kryshchyshyn, A., Kaminskyy, D., Karpenko, O., Gzella, A., Grellier, P., Lesyk, R. (2019). Thiazolidinone/thiazole based hybrids – New class of antitrypanosomal agents. European Journal of Medicinal Chemistry, 174, 292–308. https://doi.org/10.1016/j.ejmech.2019.04.052
- Kryshchyshyn, A., Kaminskyy, D., Nektegayev, I., Grellier, P., Lesyk, R. (2018). Isothiochromenothiazoles – A Class of Fused Thiazolidinone Derivatives with Established Anticancer Activity That Inhibits Growth of Trypanosoma brucei brucei. Scientia Pharmaceutica, 86 (4), 47. https://doi.org/10.3390/scipharm86040047
- Bhagat, D. S., Chawla, P. A., Gurnule, W. B., Shejul, S. K., Bumbrah, G. S. (2021). An Insight into Synthesis and Anticancer Potential of Thiazole and 4-thiazolidinone Containing Motifs. Current Organic Chemistry, 25 (7), 819–841. https://doi.org/10.2174/1385272825999210101234704
- Latambale, G., Juvale, K. (2025). Thiazolidinedione derivatives: emerging role in cancer therapy. Molecular Diversity. https://doi.org/10.1007/s11030-024-11093-3
- Iqbal, M. A., Husain, A., Alam, O., Khan, S. A., Ahmad, A., Haider, M. R., Alam, M. A. (2020). Design, synthesis, and biological evaluation of imidazopyridine‐linked thiazolidinone as potential anticancer agents. Archiv Der Pharmazie, 353 (10). https://doi.org/10.1002/ardp.202000071
- Türe, A., Ergül, M., Ergül, M., Altun, A., Küçükgüzel, İ. (2020). Design, synthesis, and anticancer activity of novel 4-thiazolidinone-phenylaminopyrimidine hybrids. Molecular Diversity, 25 (2), 1025–1050. https://doi.org/10.1007/s11030-020-10087-1
- Kryshchyshyn-Dylevych, A., Radko, L., Finiuk, N., Garazd, M., Kashchak, N., Posyniak, A. et al. (2021). Synthesis of novel indole-thiazolidinone hybrid structures as promising scaffold with anticancer potential. Bioorganic & Medicinal Chemistry, 50, 116453. https://doi.org/10.1016/j.bmc.2021.116453
- Ansari, M. F., Idrees, D., Hassan, Md. I., Ahmad, K., Avecilla, F., Azam, A. (2018). Design, synthesis and biological evaluation of novel pyridine-thiazolidinone derivatives as anticancer agents: Targeting human carbonic anhydrase IX. European Journal of Medicinal Chemistry, 144, 544–556. https://doi.org/10.1016/j.ejmech.2017.12.049
- Tokalı, F. S., Şenol, H., Katmerlikaya, T. G., Dağ, A., Şendil, K. (2023). Novel thiosemicarbazone and thiazolidin‐4‐one derivatives containing vanillin core: Synthesis, characterization, and anticancer activity studies. Journal of Heterocyclic Chemistry, 60 (4), 645–656. https://doi.org/10.1002/jhet.4619
- Paneth, A., Kaproń, B., Plech, T., Paduch, R., Trotsko, N., Paneth, P. (2023). Combined In Silico and In Vitro Analyses to Assess the Anticancer Potential of Thiazolidinedione–Thiosemicarbazone Hybrid Molecules. International Journal of Molecular Sciences, 24 (24), 17521. https://doi.org/10.3390/ijms242417521
- Souza Tada da Cunha, P., Rodriguez Gini, A. L., Man Chin, C., dos Santos, J. L., Benito Scarim, C. (2025). Recent Progress in Thiazole, Thiosemicarbazone, and Semicarbazone Derivatives as Antiparasitic Agents Against Trypanosomatids and Plasmodium spp. Molecules, 30 (8), 1788. https://doi.org/10.3390/molecules30081788
- de Aquino, T. M., França, P. H. B., Rodrigues, É. E. E. S., Nascimento, Igor. J. S., Santos-Júnior, P. F. S. et al. (2022). Synthesis, Antileishmanial Activity and in silico Studies of Aminoguanidine Hydrazones (AGH) and Thiosemicarbazones (TSC) Against Leishmania chagasi Amastigotes. Medicinal Chemistry, 18 (2), 151–169. https://doi.org/10.2174/1573406417666210216154428
- Scarim, C. B., Jornada, D. H., Machado, M. G. M., Ferreira, C. M. R., dos Santos, J. L., Chung, M. C. (2019). Thiazole, thio and semicarbazone derivatives against tropical infective diseases: Chagas disease, human African trypanosomiasis (HAT), leishmaniasis, and malaria. European Journal of Medicinal Chemistry, 162, 378–395. https://doi.org/10.1016/j.ejmech.2018.11.013
- Pays, E., Radwanska, M., Magez, S. (2023). The Pathogenesis of African Trypanosomiasis. Annual Review of Pathology: Mechanisms of Disease, 18 (1), 19–45. https://doi.org/10.1146/annurev-pathmechdis-031621-025153
- Kryshchyshyn, A., Kaminskyy, D., Grellier, P., Lesyk, R. (2021). Thiazolidinone-Related Heterocyclic Compounds as Potential Antitrypanosomal Agents. Azoles – Synthesis, Properties, Applications and Perspectives. https://doi.org/10.5772/intechopen.91861
- Chagas disease (American trypanosomiasis). World Health Organization. Available at: https://www.who.int/health-topics/chagas-disease Last accessed: 05.05.2025
- Jiang, J., Yu, Y. (2024). Eflornithine for treatment of high-risk neuroblastoma. Trends in Pharmacological Sciences, 45 (6), 577–578. https://doi.org/10.1016/j.tips.2024.04.005
- Shakeel, A., Baloch, A., Kumari, V., Kazmi, S. K. Z., Aftab, K., Abid, S. et al. (2024). Iwilfin (eflornithine) approved by the FDA as the first and only oral maintenance therapy for high-risk neuroblastoma in adult and pediatric patients: Narrative review. Medicine, 103 (48), e40662. https://doi.org/10.1097/md.0000000000040662
- Li, Y.-Q., Zheng, Z., Liu, Q.-X., Lu, X., Zhou, D., Zhang, J. et al. (2021). Repositioning of Antiparasitic Drugs for Tumor Treatment. Frontiers in Oncology, 11. https://doi.org/10.3389/fonc.2021.670804
- Mou, Y., Wang, J., Wu, J., He, D., Zhang, C., Duan, C., Li, B. (2019). Ferroptosis, a new form of cell death: opportunities and challenges in cancer. Journal of Hematology & Oncology, 12 (1). https://doi.org/10.1186/s13045-019-0720-y
- Huang, H., He, Q., Guo, B., Xu, X., Wu, Y., Li, X. (2021). Progress in Redirecting Antiparasitic Drugs for Cancer Treatment. Drug Design, Development and Therapy, 15, 2747–2767. https://doi.org/10.2147/dddt.s308973
- Holota, S., Kryshchyshyn, A., Derkach, H., Trufin, Y., Demchuk, I., Gzella, A. et al. (2019). Synthesis of 5-enamine-4-thiazolidinone derivatives with trypanocidal and anticancer activity. Bioorganic Chemistry, 86, 126–136. https://doi.org/10.1016/j.bioorg.2019.01.045
- Kryshchyshyn-Dylevych, A. P., Zelisko, N. I., Grellier, P., Lesyk, R. B. (2020). Preliminary evaluation of thiazolidinone- and pyrazoline-related heterocyclic derivatives as potential antimalarial agents. Biopolymers and Cell, 36 (1), 48–60. https://doi.org/10.7124/bc.000a20
- Kryshchyshyn-Dylevych, A. (2020). Some pharmacological properties of 4-[3-(5-bromo-2-hydroxyphenyl)-5-phenyl-3,4-dihydropyrazol-2-yl]-5H-thiazol-2-one. Ukrainica Bioorganica Acta, 15 (2), 41–48. https://doi.org/10.15407/bioorganica2020.02.041
- Bastos, I. M. D., Motta, F. N., Charneau, S., Santana, J. M., Dubost, L., Augustyns, K., Grellier, P. (2010). Prolyl oligopeptidase of Trypanosoma brucei hydrolyzes native collagen, peptide hormones and is active in the plasma of infected mice. Microbes and Infection, 12 (6), 457–466. https://doi.org/10.1016/j.micinf.2010.02.007
- Lethu, S., Bosc, D., Mouray, E., Grellier, P., Dubois, J. (2012). New protein farnesyltransferase inhibitors in the 3-arylthiophene 2-carboxylic acid series: diversification of the aryl moiety by solid-phase synthesis. Journal of Enzyme Inhibition and Medicinal Chemistry, 28 (1), 163–171. https://doi.org/10.3109/14756366.2011.643302
- Boyd, M. R., Paull, K. D. (1995). Some practical considerations and applications of the national cancer institute in vitro anticancer drug discovery screen. Drug Development Research, 34 (2), 91–109. https://doi.org/10.1002/ddr.430340203
- Shoemaker, R. H. (2006). The NCI60 human tumour cell line anticancer drug screen. Nature Reviews Cancer, 6 (10), 813–823. https://doi.org/10.1038/nrc1951
- MacQueen, J. (1967). Some methods for classification and analysis of multivariate observations. Proceedings of the Fifth Berkeley Symposium on Mathematical Statistics and Probability, Volume 1: Statistics University of California press, 5, 281–298.
- Hartigan, J. A., & Wong, M. A. (1979). Algorithm AS 136: A K-Means Clustering Algorithm. Journal of the Royal Statistical Society. Series C (Applied Statistics), 28 (1), 100–108. https://doi.org/10.2307/2346830
- Ward, J. H. (1963). Hierarchical Grouping to Optimize an Objective Function. Journal of the American Statistical Association, 58 (301), 236–244. https://doi.org/10.1080/01621459.1963.10500845
- Wolber, G., Langer, T. (2005). LigandScout: 3-D Pharmacophores Derived from Protein-Bound Ligands and Their Use as Virtual Screening Filters. Journal of Chemical Information and Modeling, 45 (1), 160–169. https://doi.org/10.1021/ci049885e
- Birkinshaw, R. W., Gong, J., Luo, C. S., Lio, D., White, C. A., Anderson, M. A. et al. (2019). Structures of BCL-2 in complex with venetoclax reveal the molecular basis of resistance mutations. Nature Communications, 10 (1), 2385. https://doi.org/10.1038/s41467-019-10363-1
- Hafez, D. E., Hafez, E., Eddiasty, I., Shih, S.-P., Chien, L.-C., Hong, Y.-J. et al. (2021). Novel thiazolidine derivatives as potent selective pro-apoptotic agents. Bioorganic Chemistry, 114, 105143. https://doi.org/10.1016/j.bioorg.2021.105143
- Helmy, S. W., Shahin, M. I., Samir, N., Lasheen, D. S., Ella, D. A. A. E. (2024). Targeting apoptosis; design, synthesis and biological evaluation of new benzoxazole and thiazole based derivatives. BMC Chemistry, 18 (1). https://doi.org/10.1186/s13065-023-01101-2
- Guerra, V. A., DiNardo, C., Konopleva, M. (2019). Venetoclax-based therapies for acute myeloid leukemia. Best Practice & Research Clinical Haematology, 32 (2), 145–153. https://doi.org/10.1016/j.beha.2019.05.008
- Nguyen, W., Lee, E. F., Evangelista, M., Lee, M., Harris, T. J., Colman, P. M. et al. (2021). Optimization of Benzothiazole and Thiazole Hydrazones as Inhibitors of Schistosome BCL-2. ACS Infectious Diseases, 7 (5), 1143–1163. https://doi.org/10.1021/acsinfecdis.0c00700
##submission.downloads##
Опубліковано
Як цитувати
Номер
Розділ
Ліцензія
Авторське право (c) 2025 Anna Kryshchyshyn-Dylevych, Roman Lesyk

Ця робота ліцензується відповідно до Creative Commons Attribution 4.0 International License.
Наше видання використовує положення про авторські права Creative Commons CC BY для журналів відкритого доступу.







