Two masterful alternative spectrophotometric methods for the determination of ramipril in tablets
DOI:
https://doi.org/10.15587/2519-4852.2024.302635Keywords:
ramipril, spectrophotometry, validation, quantitative determination, tabletsAbstract
The aim of the work was to develop two simple, rapid, economic, alternative spectrophotometric methods for the determination of ramipril in tablets based on the reaction with sulfonephthalein dyes (bromphenol blue (BPB) and cresol red (CR)).
Materials and methods. Analytical instrumentation: Shimadzu UV-1800 double beam UV-VIS spectrophotometer (Japan) with attached UV-Probe ver. 2.62 software, RAD WAG AS 200/C precise analytical balance (Poland). Ramipril (purity ≥98 % (HPLC)) was purchased from AARTI Industries Limited (India). Ramipril tablets 5 mg and 10 mg were purchased from a local pharmacy.
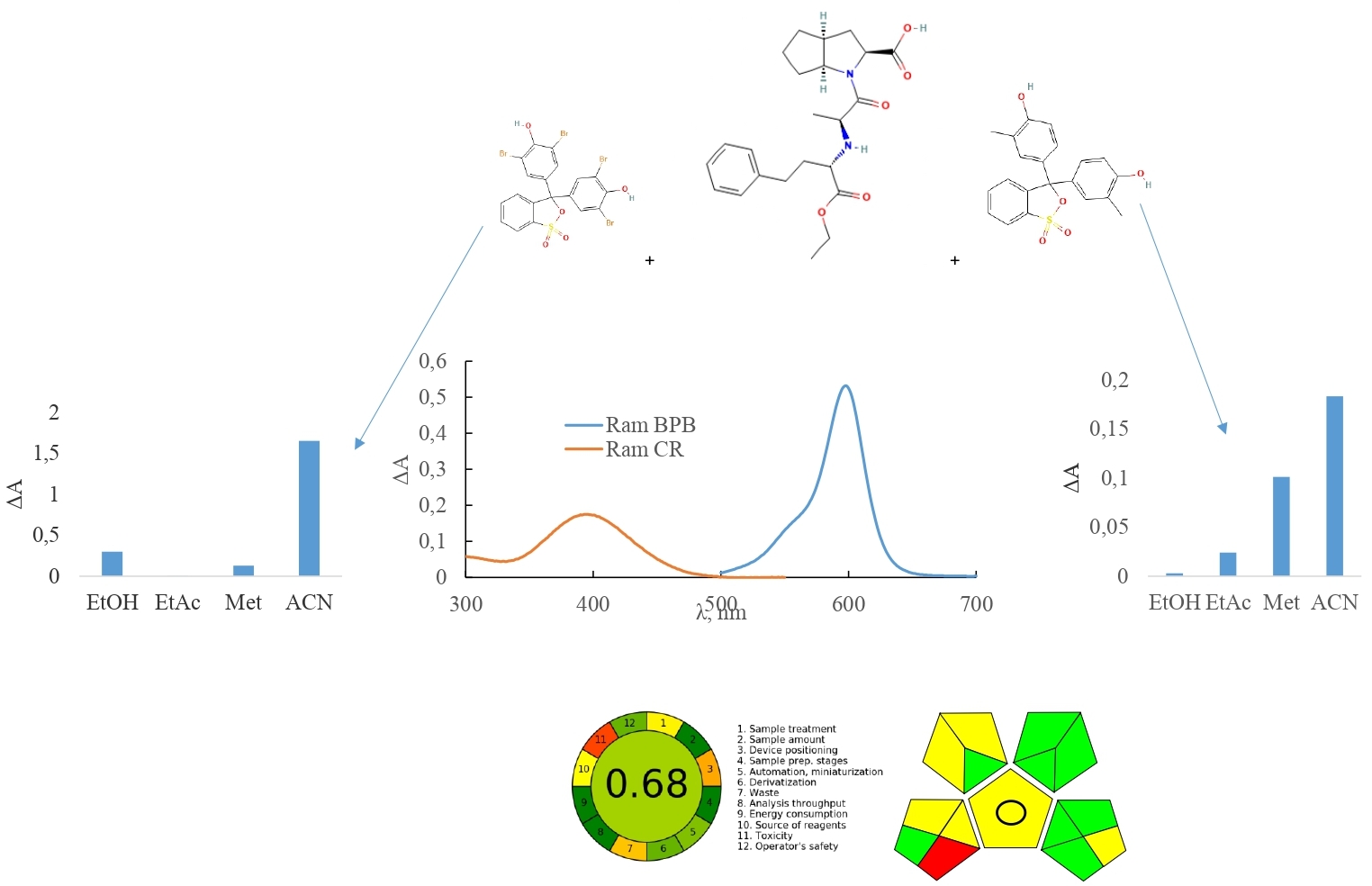
Results and discussion. Two spectrophotometric methods for the determination of ramipril in tablets have been developed. We have tested various sulfophthalein dyes (BPB, bromocresol green, bromthymol blue, thymol blue, CR) in order to choose the optimal for the method development. According to the results of the experimental studies, we chose BPB and CR as reagents, and the solvent was acetonitrile for both methods. The optimal conditions for the quantitative determination of ramipril in tabletsy using BPB were established: dye concentration - 2.35×10-4 mol/L, volume of BPB solution – 1.0 ml, without heating, wavelength – 598 nm, reaction time – 5 min, temperature of the solution – 25 °C. The optimal conditions for the quantitative determination of ramipril in tabletsusing CR were established: dye 1.33×10-4 mol/L, volume of CR solution – 1.0 ml, without heating, wavelength – 395 nm, reaction time – 5 min, temperature of the solution – 25 °C. The spectrophotometric method by using BPB was linear in the concentration range of 1.99-5.96 µg/mL, LOD – 0.20 µg/mL, LOQ – 0.60 µg/mL. The spectrophotometric method using CR was linear in the concentration range of 0.42-5.44 µg/mL, LOD – 0.10 µg/mL, and LOQ – 0.36 µg/mL. The results of the study on robustness, accuracy, and precision were within the acceptance criteria. The results of studying the «greenness» of both methods indicate an excellent «green» analysis.Conclusions. Developed methods can be used as an alternative method for the routine analysis of ramipril tablets
References
- Cardiovascular Diseases (CVDs). Available at: https://www.who.int/health-topics/cardiovascular-diseases#tab=tab_3 Last accessed: 22.03.2024
- Ramipril. Available at: https://pubchem.ncbi.nlm.nih.gov/compound/Ramipril Last accessed: 14.03.2024
- European Pharmacopoeia. 11 ed. (2022). Available at: https://www.edqm.eu/en/european-pharmacopoeia-ph.-eur.-11th-edition Last accessed: 22.03.2024
- Babu, K. A., Kumar, G. V., Sivasubramanian, L. (2011). Simultaneous estimation of ramipril and amlodipine in pharmaceutical dosage form by RP-HPLC method. International Journal of Pharmacy and Pharmaceutical Sciences, 3 (4), 196–198.
- Dai, S.-Y., Qiu, S.-T., Wu, W., Fu, C.-M. (2013). Development and validation of an RP-HPLC method for simultaneous determination of Ramipril and Amlodipine in tablets. Journal of Pharmaceutical Analysis, 3 (6), 440–446. https://doi.org/10.1016/j.jpha.2013.09.002
- Elshanawane, A. A., Mostafa, S. M., Elgawish, M. S. (2008). Application of a Validated, Stability-Indicating LC Method to Stress Degradation Studies of Ramipril and Moexipril.HCl. Chromatographia, 67 (7-8), 567–573. https://doi.org/10.1365/s10337-008-0544-3
- Gupta, K. R., Wankhede, S. B., Tajne, M. R., Wadodkar, S. G. (2007). Simultaneous determination of amlodipine and ramipril by high performance thin layer chromatography. Asian Journal of Chemistry, 19 (6), 4177–4182.
- Kumar, P. V., Nasare, M., Rao, V., Prasad, V. V. L. N., Diwan, P. V. (2012). Isocratic Reverse Phase High Performance Liquid Chromatographic Estimation of Ramipril and Amlodipine in Pharmaceutical Dosage Form. Journal of Advanced Pharmacy Education and Research, 2 (3), 137–145.
- Maste, M. M., Kalekar, M. C., Kadian, N., & Bhat, A. R. (2011). Development of RP-HPLC Method for Simultaneous Estimation of Amlodipine and Ramipril in Tablet Dosage form. Asian Journal of Research in Chemistry, 4(8), 1210-1213.
- Panchal, H. J., Suhagia, B. N., Patel, N. J., Rathod, I. S., Patel, B. H. (2008). Simultaneous Estimation of Atorvastatin Calcium, Ramipril and Aspirin in Capsule Dosage Form by RP-LC. Chromatographia, 69 (1-2), 91–95. https://doi.org/10.1365/s10337-008-0831-z
- Patel, J., Patel, M. (2014). RP-HPLC method development and validation for the simultaneous estimation of ramipril and amlodipine besylate in capsule dosage form. Journal of Chemical and Pharmaceutical Research, 6 (6), 725–733.
- Damle, M. S., Patole, S. M., Potale, L. V., Khodke, A. S. (2010). A validated HPLC method for analysis of atorvastatin calcium, ramipril and aspirin as the bulk drug and in combined capsule dosage Forms. International Journal of Pharmaceutical Sciences Review and Research, 4 (3), 40–45.
- Rajput, P. S., Kaur, A., Gill, N. K., Mittal, K., Sarma, G. S. (2012). Simultaneous estimation of ramipril and amlodipine in bulk and tablet dosage form by RP-HPLC method. Journal of Applied Pharmaceutical Science, 2 (7), 160–165. https://doi.org/10.7324/japs.2012.2724
- Dheeravath, S. N., Ramadevi, K., Saraswathi, Z., Maniklal, D., Bhagawan. D, Bhagawan. D. (2012). RP-HPLC method development for simultaneous determination of the drugs Ramipril and Amlodipine. International Journal of Scientific Research, 2 (2), 364–367. https://doi.org/10.15373/22778179/feb2013/123
- Sharma, R., Khanna, S., Mishra, G. P. (2012). Development and Validation of RP-HPLC Method for Simultaneous Estimation of Ramipril, Aspirin and Atorvastatin in Pharmaceutical Preparations. E-Journal of Chemistry, 9 (4), 2177–2184. https://doi.org/10.1155/2012/891695
- Rao, S., Srinivas, K. (2010). RP-HPLC method for the determination of losartan potassium and ramipril in combined dosage form. Indian Journal of Pharmaceutical Sciences, 72 (1), 108–111. https://doi.org/10.4103/0250-474x.62243
- Joseph, L., Mathew, G., Rao, V. R. (2008). Simultaneous estimation of atorvastatin and ramipril by RP-HPLC and spectroscopy. Pakistan journal of pharmaceutical sciences, 21 (3), 282–284.
- Sharma, A. K., Shah, B., Patel, B. (2010). Simultaneous estimation of Atorvastatin calcium, Ramipril and Aspirin in capsule dosage form using HPTLC. Der Pharma Chemica, 2 (4), 10–16.
- Żuromska-Witek, B., Stolarczyk, M., Szlósarczyk, M., Kielar, S., Hubicka, U. (2022). Simple, Accurate and Multianalyte Determination of Thirteen Active Pharmaceutical Ingredients in Polypills by HPLC-DAD. Chemosensors, 11(1), 25. https://doi.org/10.3390/chemosensors11010025
- De Diego, M., Godoy, G., Mennickent, S., Olivares, M., Godoy, R. (2010). Stress degradation studies of ramipril by a validated stability-indicating liquid chromatographic method. Journal of the Chilean Chemical Society, 55 (4), 450–453. https://doi.org/10.4067/s0717-97072010000400008
- Typlynska, K., Kondratova, Y., Logoyda, L. (2023). Development of Methods of Quality Control of the Tablets «Ramipril». Scientia Pharmaceutica, 91 (2), 21. https://doi.org/10.3390/scipharm91020021
- The United States Pharmacopeia; The National Formulary (2021). Rockville: United States Pharmacopeial Convention, Inc. Available at: https://www.uspnf.com Last accessed: 22.03.2024
- Al-Majed, A. A., Belal, F., Al-Warthan, A. A. (2001). Spectrophotometric determination of ramipril (a novel ACE inhibitor) in dosage forms. Spectroscopy Letters, 34 (2), 211–220. https://doi.org/10.1081/sl-100002010
- Rahman, N., Ahmad, Y., Azmi, S. N. H. (2005). Kinetic spectrophotometric method for the determination of ramipril in pharmaceutical formulations. AAPS PharmSciTech, 6 (3), E543–E551. https://doi.org/10.1208/pt060368
- Salem, H. (1999). Derivative spectrophotometric determination of two component mixtures. Zhonghuá yáoxué zázhì, 51 (2), 123–142.
- Afieroho, O., Okorie, O., Okonkwo, T. (2012). A Spectrophotometric Method for the Determination of Ramipril in Solid Dosage Forms. Tropical Journal of Pharmaceutical Research, 11 (2), 275–279. https://doi.org/10.4314/tjpr.v11i2.15
- Attimarad, M., Venugopala, K. N., Aldhubiab, B. E., Nair, A. B., SreeHarsha, N., Pottathil, S., Akrawi, S. H. (2019). Development of UV Spectrophotometric Procedures for Determination of Amlodipine and Celecoxib in Formulation: Use of Scaling Factor to Improve the Sensitivity. Journal of Spectroscopy, 2019, 1–10. https://doi.org/10.1155/2019/8202160
- Kumar, M., Jindal, M., Bhatt, S., Pandurangan, A., Malik, A., Kaushik, V. et al. (2019). Simultaneous estimation of amlodipine besylate and ramipril in tablets dosage form by UV spectrophotometric method. Journal of Pharmaceutical Sciences and Research, 11 (2), 667–670.
- Patel, A. B., Jadav, H. M., Vyas, A. J., Patel, A. I., Patel, N. K., Chudasama, A. (2020). Simultaneous determination of ramipril and amlodipine besylate in tablet dosage form by first order derivative spectrophotometric method. Chemical Methodologies, 4 (4), 467–476. https://doi.org/10.33945/sami/chemm.2020.4.8
- Attala, K., Elsonbaty, A. (2021). Smart UV spectrophotometric methods based on simple mathematical filtration for the simultaneous determination of celecoxib and ramipril in their pharmaceutical mixtures with amlodipine: A comparative statistical study. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 244, 118853. https://doi.org/10.1016/j.saa.2020.118853
- Shulyak, N., Protsyk, S., Kuche, T., Kryskiw, L., Poliak, O., Mosula, L., Logoyda, L. (2022). Development of the Spectrophotometric Method for the Determination of Atorvastatin Calcium in Tablets by using Bromophenol Blue. Methods and Objects of Chemical Analysis, 17 (3), 111–117. https://doi.org/10.17721/moca.2022.111-117
- Bromophenol Blue (Compound). 2D Structure. Available at: https://pubchem.ncbi.nlm.nih.gov/compound/Bromophenol-Blue#section=2D-Structure
- Cresol red. Available at: https://pubchem.ncbi.nlm.nih.gov/compound/Cresol-red
- Green Solvent Selection Tool. Available at: https://green-solvent-tool.herokuapp.com/
- State Pharmacopoeia of Ukraine. Vol. 1 (2015). Kharkiv: SE “Ukrainian Scientific Pharmacopoeial Center for Quality of Medicines”, 11148.
- Ermer, J., Agut, C. (2014). Precision of the reportable result. Simultaneous optimisation of number of preparations and injections for sample and reference standard in quantitative liquid chromatography. Journal of Chromatography A, 1353, 71–77. https://doi.org/10.1016/j.chroma.2014.03.043
- Pena-Pereira, F., Wojnowski, W., Tobiszewski, M. (2020). AGREE – Analytical GREEnness Metric Approach and Software. Analytical Chemistry, 92 (14), 10076–10082. https://doi.org/10.1021/acs.analchem.0c01887
- Płotka-Wasylka, J. (2018). A new tool for the evaluation of the analytical procedure: Green Analytical Procedure Index. Talanta, 181, 204–209. https://doi.org/10.1016/j.talanta.2018.01.013
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2024 Kateryna Typlynska, Mariana Horyn, Tetyana Kucher, Liubomyr Kryskiw, Liliya Logoyda

This work is licensed under a Creative Commons Attribution 4.0 International License.
Our journal abides by the Creative Commons CC BY copyright rights and permissions for open access journals.






