Development of a method for the quantitative determination of the solubility limits of poorly soluble in water substances on the example of quercetin
DOI:
https://doi.org/10.15587/2519-4852.2023.283293Keywords:
quercetin, identification, quantitative definition, HPLC, method development, bioequivalence, biowaiver, mass spectrometry, solubility, dissolution testingAbstract
Aim. To consider the importance of this physicochemical characteristic as a determining factor in the study of bioequivalence and bioavailability, there is a need to develop a method to quantitatively determine the solubility limit of quercetin.
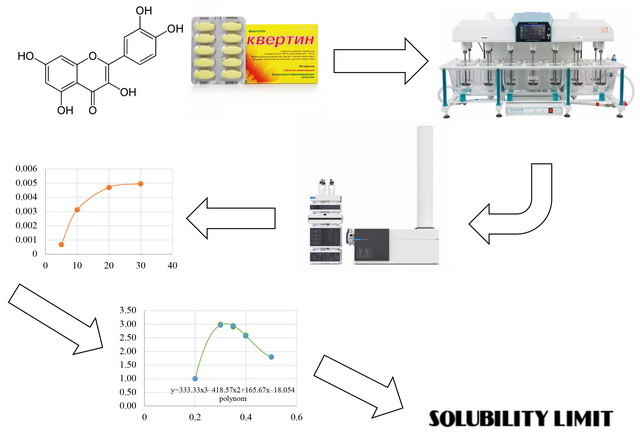
Materials and methods. The quercetin concentration was determined in the obtained samples using chromatographic and external standard methods. The pharmacopeial standard – PS of the SPU was used as a standard. For measurements, an Agilent 1290 liquid chromatograph with an Agilent 6530 TOF mass spectrometric detector was used, using a 50×4.6 mm column filled with a sorbent with a grafted phase of octyl silica gel, particle size – 1.7 μm.
Results. The exact limit of the solubility of quercetin, as a poorly soluble substance, has been established. Based on the data obtained, the kinetics of the dissolution of quercetin was studied. In tandem with the QTOF mass spectrometric detector, the HPLC method was utilized in the identification and quantification process. To accurately determine the point that will correspond to the solubility limit of quercetin in water, the obtained experimental dependence was approximated by a polynomial dependence, for which, by solving a system of equations in the Microsoft Excel program, concentration values were found corresponding to the inflection points of the studied dependences.
Conclusions. When studying their bioavailability, a new approach has been developed to quantitatively determine the solubility limit of difficult or practically insoluble substances in aqueous media with a neutral pH value. The exact value of the solubility limit for the test sample of quercetin was established, which was 3.02 mcg/ml. The kinetics of the release of quercetin in aqueous solutions was studied
References
- Mudrak, I. H., Zaliska, O. M. (2011). Analiz dynamiky dokazovykh danykh pro likarski roslynni zasoby u bazi Kokrana. Farmatsevtychnyi chasopys, 3, 75–78.
- Sahoo, N., Manchikanti, P., Dey, S. (2010). Herbal drugs: Standards and regulation. Fitoterapia, 81 (6), 462–471. doi: https://doi.org/10.1016/j.fitote.2010.02.001
- Borghetti, G. S., Carini, J. P., Honorato, S. B., Ayala, A. P., Moreira, J. C. F., Bassani, V. L. (2012). Physicochemical properties and thermal stability of quercetin hydrates in the solid state. Thermochimica Acta, 539, 109–114. doi: https://doi.org/10.1016/j.tca.2012.04.015
- Kaşıkcı, M., Bağdatlıoğlu, N. (2016). Bioavailability of Quercetin. Current Research in Nutrition and Food Science Journal, 4 (2), 146–151. doi: https://doi.org/10.12944/crnfsj.4.special-issue-october.20
- Parasuraman, S., Anand David, A., Arulmoli, R. (2016). Overviews of biological importance of quercetin: A bioactive flavonoid. Pharmacognosy Reviews, 10 (20), 84–89. doi: https://doi.org/10.4103/0973-7847.194044
- Zheng, Y.-Y., Ma, Y.-T., Zhang, J.-Y., Xie, X. (2020). COVID-19 and the cardiovascular system. Nature Reviews Cardiology, 17 (5), 259–260. doi: https://doi.org/10.1038/s41569-020-0360-5
- Althans, D., Schrader, P., Enders, S. (2014). Solubilisation of quercetin: Comparison of hyperbranched polymer and hydrogel. Journal of Molecular Liquids, 196, 86–93. doi: https://doi.org/10.1016/j.molliq.2014.03.028
- WHO Model List of Essential Medicines, 18th list (2013). World Health Organization. Available at: https://apps.who.int/iris/handle/10665/93142
- Abraham, M. H., Acree, W. E. (2014). On the solubility of quercetin. Journal of Molecular Liquids, 197, 157–159. doi: https://doi.org/10.1016/j.molliq.2014.05.006
- Aguda, R., Chen, C.-C. (2016). Solubility of Nutraceutical Compounds in Generally Recognized as Safe Solvents at 298 K. International Journal of Chemical Engineering and Applications, 7 (5), 289–294. doi: https://doi.org/10.18178/ijcea.2016.7.5.591
- NCBI homepage U.S. National Library of Medicine National Center for Biotechnology Information. Pubchem. Available at: https://pubchem.ncbi.nlm.nih.gov/
- Chebil, L., Chipot, C., Archambault, F., Humeau, C., Engasser, J. M., Ghoul, M., Dehez, F. (2010). Solubilities Inferred from the Combination of Experiment and Simulation. Case Study of Quercetin in a Variety of Solvents. The Journal of Physical Chemistry B, 114 (38), 12308–12313. doi: https://doi.org/10.1021/jp104569k
- Srinivas, K., King, J. W., Howard, L. R., Monrad, J. K. (2010). Solubility and solution thermodynamic properties of quercetin and quercetin dihydrate in subcritical water. Journal of Food Engineering, 100 (2), 208–218. doi: https://doi.org/10.1016/j.jfoodeng.2010.04.001
- State Pharmacopoeia of Ukraine. Vol. 1 (2015). Kharkiv: Ukrainian Scientific Pharmacopoeial Center for Quality of Medicines, 1128.
- European Pharmacopoeia (2016). Strasbourg: European Department for the Quality of Medicines.
- Nastanova z klinichnykh doslidzhen. Likarski zasoby. Doslidzhennia biodostupnosti ta bioekvivalentnosti. Nastanova 42–7.1:2005. Kyiv: Ministerstvo okhorony zdorov’ia Ukrainy.
- M9 Biopharmaceutics Classification System-Based Biowaivers, Guidance for Industry U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER) Center for Biologics Evaluation and Research (CBER) (2021). Available at: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/m9-biopharmaceutics-classification-system-based-biowaivers
- Marchyshyn, S. M., Stoiko, L. I., Mosula, L. M. (2018). Vyznachennia flavonoidiv tyrlychu khreshchatoho travy (Gentiana Cruciata l.). Fitoterapiia. Chasopys, 2, 58–61.
- Grizodub, A. I., Gubarevich, I. G., Nikishina, L. E., Leontev, D. A., Akichev, A. Sh., Glumenko, E. N., Baumer, V. N. (2008). Zavisimost rastvorimosti fensuktcinala ot razmera chastitc. Farmakom, 1, 50–67.
- Doerfel, K. (1968). Statystyka v analytychnoi khimii. Moscow: Mir.
- Epshtein, N. A. (2019). Validation of Analytical Procedures: Graphic and Calculated Criteria for Assessment of Methods Linearity in Practice. Drug Development & Registration, 8 (2), 122–130. doi: https://doi.org/10.33380/2305-2066-2019-8-2-122-130
- Kovalevska, I. V., Ruban, O. A., Hrudko, V. O. (2015). Study of quercetin release of solid dispersions with macromolecular substances. Zbirnyk naukovykh prats spivrobitnykiv NMAPO im. P. L. Shupyka, 24 (5), 318–322. Available at: http://nbuv.gov.ua/UJRN/Znpsnmapo_2015_24%285%29__62
- Zenkevich, I. G., Guschina, S. V. (2010). Determination of dissociation constants of species oxidizable in aqueous solution by air oxygen on an example of quercetin. Journal of Analytical Chemistry, 65 (4), 371–375. doi: https://doi.org/10.1134/s1061934810040064
- Zenkevich, I. G., Olisov, D. A., Shafigulin, R. V., Bulanova, A. V. (2019). A new approach to the chromatographic determination of quercetin water solubility. Analitika i Kontrol, 23 (3), 386–392. doi: https://doi.org/10.15826/analitika.2019.23.3.013
- Kovalevska, I. V. (2014). Quercetin physical-chemical characteristics’ definition. Current issues in pharmacy and medicine: science and practice, 1 (14): 9–11. Available at: http://nbuv.gov.ua/UJRN/apfimntp_2014_1_5
- Shubenkova, E. G. (2015). Kolloidnaia khimiia. Poverkhnostnye iavleniia: praktikum. Omsk: Izd-vo OmGTU, 64.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2023 Nataliia Khanina, Victoriya Georgiyants, Vadim Khanin

This work is licensed under a Creative Commons Attribution 4.0 International License.
Our journal abides by the Creative Commons CC BY copyright rights and permissions for open access journals.






