Computer-aided rational design and synthesis of new potential antihypertensive agents among 1,2,3-triazole-containing nifedipine analogs
DOI:
https://doi.org/10.15587/2519-4852.2024.291626Keywords:
1,4-dihydropyridine, 1,2,3-triazole, calcium channel blockers, antihypertensive agents, molecular docking, scaling up of the synthesis methodAbstract
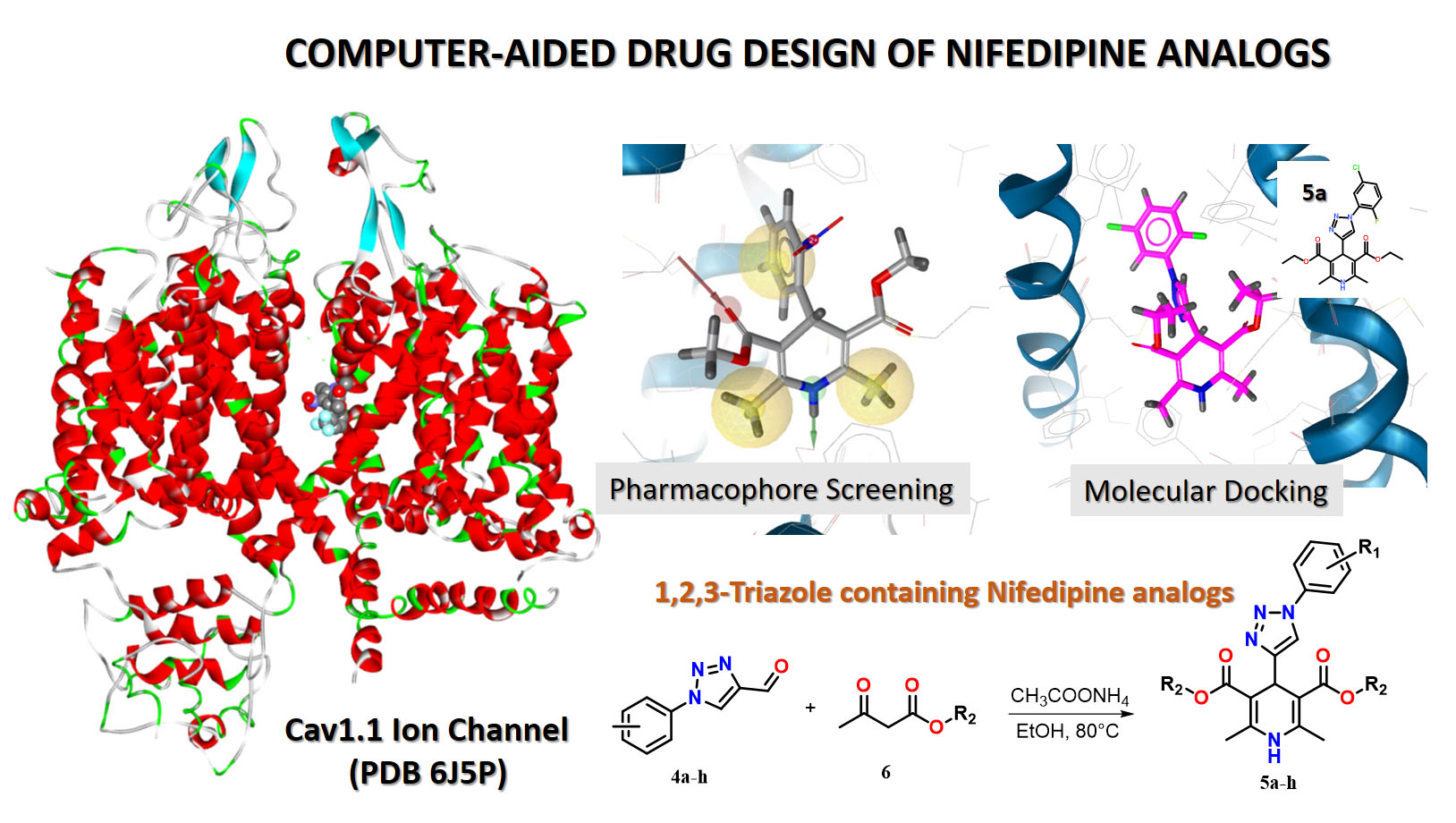
1,2,3-Triazole-containing Nifedipine analogues offer the opportunity to increase biostability, bioavailability, efficacy and binding selectivity to target receptors. Here, we applied a computer-aided rational design for identifying new Nifedipine analogues containing a 1,2,3-triazole moiety. First, a new chemical library of 796 derivatives combining the DHP fragment and 1,2,3-triazole moiety was generated. Second, to reduce the library size, the library was pre-filtered using two 3D-pharmacophore models with different complexity, which allowed us to gradually reduce the chemical space, ending up with 26 hit candidates. Molecular docking calculations against the rCav1.1 receptor allowed the identification of eight derivatives 5a-h, characterized by the binding affinity towards the rCav1.1 receptor of the same level as approved Nifedipine-like drugs. Next, our molecular docking results were used to guide and optimize the retrosynthetic approaches for new analogues of Nifedipine as promising antihypertensive agents. So, a retrosynthetic approach for Nifedipine analogues with a 1,2,3-triazole ring in position 4 was proposed. Finally, eight analogues 5a-h determined by molecular docking calculations were synthesized using the suggested retrosynthetic approach.
The aim of this study is to identify new Nifedipine analogues using a computer-aided drug design and a retrosynthetic approach.
Materials and Methods. The organic synthesis of new Nifedipine analogues containing a 1,2,3-triazole moiety. Computer-aided drug design of new DHP derivatives using pharmacophore screening and molecular docking calculations.
Results. Molecular docking of new Nifedipine analogues made it possible to estimate the binding affinity of new Nifedipine derivatives to the rCav1.1 receptor. Pharmacophore screening of a chemical library of analogues, consisting of 796 derivatives, allowed gradually reducing the chemical space and obtaining 26 candidates with high affinity to the rCav1.1 receptor. Using the method of molecular docking, eight hits 5a-h were identified, and the synthesis of the recommended compounds was proposed and performed.
Conclusions. The results of molecular docking showed that Nifedipine analogues are characterized by binding affinity to the rCav1.1 receptor at the same level as approved Nifedipine-like drugs. Pharmacophore screening and molecular docking calculations indicate key features of the ligand-receptor interaction that can guide and optimize the synthesis of new Nifedipine analogues as promising new antihypertensive agents. A retrosynthetic approach was proposed, and the recommended compounds were synthesized
Supporting Agency
- This project has received funding through the MSCA4Ukraine project, which is funded by the European Union. Views and opinions expressed are however those of the author(s) only and do not necessarily reflect those of the European Union. Neither the European Union nor the MSCA4Ukraine Consortium as a whole nor any individual member institutions of the MSCA4Ukraine Consortium can be held responsible for them
References
- Petkova, V., Peneva, A., Andreevska, K., Hristov, E., Grekova, D., Todorova, A. et al. (2023). Level of hypertension treatment adherence during pandemic. Pharmacia, 70 (3), 643–648. https://doi.org/10.3897/pharmacia.70.e109440
- Khedkar, S., Auti, P. (2014). 1, 4-Dihydropyridines: A Class of Pharmacologically Important Molecules. Mini-Reviews in Medicinal Chemistry, 14 (3), 282–290. https://doi.org/10.2174/1389557513666131119204126
- Parthiban, A., Parameshwar, M. (2022). 1,4-Dihydropyridine: synthetic advances, medicinal and insecticidal properties. RSC Advances, 12 (45), 29253–29290. https://doi.org/10.1039/d2ra04589c
- Wang, A. L., Iadecola, C., Wang, G. (2017). New generations of dihydropyridines for treatment of hypertension. Journal of Geriatric Cardiology, 14 (1), 67–72. https://doi.org/10.11909/j.issn.1671-5411.2017.01.006
- Zhang, Y. H., Zhang, Z. Q., Wu, Q. (1991). Synthesis of alkyl 2,6-dimethyl-(substituted or unsubstituted furyl)-1,4-dihydropyridine-3,5-dicarboxylates. Acta pharmaceutica Sinica, 26 (5), 375–378.
- Caignan, G. A., Metcalf, S. K., Holt, E. M. (2000). Thiophene substituted dihydropyridines. Journal of Chemical Crystallography, 30 (6), 415–422. https://doi.org/10.1023/a:1009538107356
- Lavilla, R., Gotsens, T., Santano, M. C., Bosch, J., Camins, A., Arnau, N. et al. (1997). Synthesis and Calcium Channel Blocking Activity of 4-Indolyl-1,4-dihydropyridines. Bioorganic Chemistry, 25 (3), 169–178. https://doi.org/10.1006/bioo.1997.1059
- Shafiee, A., Dehpour, A. R., Hadizadeh, F., Azimi, M. (1998). Syntheses and calcium channel antagonist activity of nifedipine analogues with methylsulfonylimidazolyl substituent. Pharmaceutica Acta Helvetiae, 73 (2), 75–79. https://doi.org/10.1016/s0031-6865(98)00004-1
- Nekooeian, A., Fard, S. H., Miri, R. (2016). Antihypertensive effects of new dihydropyridine derivatives on phenylephrine-raised blood pressure in rats. Research in Pharmaceutical Sciences, 11 (6), 497–504. https://doi.org/10.4103/1735-5362.194897
- Caignan, G. A., Holt, E. M. (2002). New 1,4-dihydropyridine derivatives with hetero and saturated B rings. Journal of Chemical Crystallography, 32 (9), 315–323. https://doi.org/10.1023/a:1020209608962
- Dostal, W., Heinisch, G., Holzer, W., Perhauc, I., Zheng, C. (1990). Pyridazines. LI. On the Reactivity of Pyridazine‐carbaldehydes towards Selected Active‐Hydrogen Compounds. Journal of Heterocyclic Chemistry, 27 (5), 1313–1321. https://doi.org/10.1002/jhet.5570270526
- Saini, K. K., Rani, R., Muskan, Khanna, N., Mehta, B., Kumar, R. (2023). An Overview of Recent Advances in Hantzsch’s Multicomponent Synthesis of 1,4- Dihydropyridines: A Class of Prominent Calcium Channel Blockers. Current Organic Chemistry, 27 (2), 119–129. https://doi.org/10.2174/1385272827666230403112419
- Praveenkumar, E., Gurrapu, N., Kumar Kolluri, P., Yerragunta, V., Reddy Kunduru, B., Subhashini, N. J. P. (2019). Synthesis, anti-diabetic evaluation and molecular docking studies of 4-(1-aryl-1H-1, 2, 3-triazol-4-yl)-1,4-dihydropyridine derivatives as novel 11-β hydroxysteroid dehydrogenase-1 (11β-HSD1) inhibitors. Bioorganic Chemistry, 90, 103056. https://doi.org/10.1016/j.bioorg.2019.103056
- Bajaj, S. D., Mahodaya, O. A., Tekade, P. V., Patil, V. B., Kukade, S. D. (2017). Synthesis of diethyl 4-(phenyl-substituted)-2,6-dimethyl-1,4-dihydropyridine-3,5-dicarboxylates catalyzed by CoCl2/K-10 montmorillonite in water and their antimicrobial activity. Russian Journal of General Chemistry, 87 (3), 546–549. https://doi.org/10.1134/s1070363217030264
- Lentz, F., Hemmer, M., Reiling, N., Hilgeroth, A. (2016). Discovery of novel N- phenyl 1,4-dihydropyridines with a dual mode of antimycobacterial activity. Bioorganic & Medicinal Chemistry Letters, 26 (24), 5896–5898. https://doi.org/10.1016/j.bmcl.2016.11.010
- Cateni, F., Zacchigna, M., Pedemonte, N., Galietta, L. J. V., Mazzei, M. T., Fossa, P. et al. (2009). Synthesis of 4-thiophen-2′-yl-1,4-dihydropyridines as potentiators of the CFTR chloride channel. Bioorganic & Medicinal Chemistry, 17 (23), 7894–7903. https://doi.org/10.1016/j.bmc.2009.10.028
- Kumar, R. S., Idhayadhulla, A., Abdul Nasser, A. J., Selvin, J. (2011). Synthesis and anticoagulant activity of a new series of 1,4-dihydropyridine derivatives. European Journal of Medicinal Chemistry, 46 (2), 804–810. https://doi.org/10.1016/j.ejmech.2010.12.006
- Malek, R., Maj, M., Wnorowski, A., Jóźwiak, K., Martin, H., Iriepa, I. et al. (2019). Multi-target 1,4-dihydropyridines showing calcium channel blockade and antioxidant capacity for Alzheimer’s disease therapy. Bioorganic Chemistry, 91, 103205. https://doi.org/10.1016/j.bioorg.2019.103205
- Rucins, M., Gosteva, M., Belyakov, S., Sobolev, A., Pajuste, K., Plotniece, M. et al. (2015). Evaluation of Antiradical Activity and Reducing Capacity of Synthesised Bispyridinium Dibromides Obtained by Quaternisation of 4-Pyridyl-1,4-dihydropyridines with Propargyl Bromide. Australian Journal of Chemistry, 68 (1), 86–92. https://doi.org/10.1071/ch14033
- Wolber, G., Langer, T. (2004). LigandScout: 3-D Pharmacophores Derived from Protein-Bound Ligands and Their Use as Virtual Screening Filters. Journal of Chemical Information and Modeling, 45 (1), 160–169. https://doi.org/10.1021/ci049885e
- Trott, O., Olson, A. J. (2009). AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. Journal of Computational Chemistry, 31 (2), 455–461. https://doi.org/10.1002/jcc.21334
- Humphrey, W., Dalke, A., Schulten, K. (1996). VMD: Visual molecular dynamics. Journal of Molecular Graphics, 14 (1), 33–38. https://doi.org/10.1016/0263-7855(96)00018-5
- Zhao, Y., Huang, G., Wu, J., Wu, Q., Gao, S., Yan, Z. et al. (2019). Molecular Basis for Ligand Modulation of a Mammalian Voltage-Gated Ca2+ Channel. Cell, 177 (6), 1495–1506.e12. https://doi.org/10.1016/j.cell.2019.04.043
- Dolphin, A. C. (2006). A short history of voltage‐gated calcium channels. British Journal of Pharmacology, 147 (S1), S56–S62. https://doi.org/10.1038/sj.bjp.0706442
- Lohachova, K. O., Sviatenko, A. S., Kyrychenko, A., Ivanov, V. V., Langer, T., Kovalenko, S. M., Kalugin, O. N. (2024). Computer-aided drug design of novel nirmatrelvir analogs inhibiting main protease of Coronavirus SARS-CoV-2. Journal of Applied Pharmaceutical Science, 14 (5), 232–239. https://doi.org/10.7324/japs.2024.158114
- Sander, T., Freyss, J., von Korff, M., Rufener, C. (2015). DataWarrior: An Open-Source Program For Chemistry Aware Data Visualization And Analysis. Journal of Chemical Information and Modeling, 55 (2), 460–473. https://doi.org/10.1021/ci500588j
- Li, W., Zhou, X., Luan, Y., Wang, J. (2015). Direct access to 1,4-disubstituted 1,2,3-triazoles through organocatalytic 1,3-dipolar cycloaddition reaction of α,β-unsaturated esters with azides. RSC Advances, 5 (108), 88816–88820. https://doi.org/10.1039/c5ra19038j
- Yu, D., Hu, F., Zhang, Y., Zheng, X., Kuang, C., Yang, Q. (2013). Synthesis and Biological Activity of Novel Deoxynojirimycin Derivatives as Potent α-Glucosidase Inhibitors. Zeitschrift Für Naturforschung B, 68 (4), 383–390. https://doi.org/10.5560/znb.2013-2318
- Calvino-Casilda, V., Martín-Aranda, R. M. (2020). Ordered mesoporous molecular sieves as active catalyts for the synthesis of 1,4-dihydropyridine derivatives. Catalysis Today, 354, 44–50. https://doi.org/10.1016/j.cattod.2019.06.046
- Siddiqui, M. M., Nagargoje, A. A., Raza, A. K., Pisal, P. M., Shingate, B. B. (2022). [Et3NH][HSO4] catalyzed solvent‐free synthesis of new 1,2,3‐triazolidene‐indolinone derivatives. Journal of Heterocyclic Chemistry, 59 (5), 899–908. https://doi.org/10.1002/jhet.4429
- Deshmukh, T. R., Krishna, V. S., Sriram, D., Sangshetti, J. N., Shingate, B. B. (2019). Synthesis and bioevaluation of α,α’-bis(1H-1,2,3-triazol-5-ylmethylene) ketones. Chemical Papers, 74 (3), 809–820. https://doi.org/10.1007/s11696-019-00908-5
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2024 Alexander Kyrychenko, Igor Bylov, Anna Geleverya, Sergiy Kovalenko, Iryna Zhuravel, Volodymyr Fetyukhin, Thierry Langer

This work is licensed under a Creative Commons Attribution 4.0 International License.
Our journal abides by the Creative Commons CC BY copyright rights and permissions for open access journals.






