CYP2C19 polymorphisms on escitalopram treatment outcome in South Indian population with major depressive disorder
DOI:
https://doi.org/10.15587/2519-4852.2024.307289Keywords:
Major Depressive Disorder, efficacy, safety, escitalopram, genotype, phenotypeAbstract
Various CYP2C19-mediated metabolizer groups may arise as a result of inter-individual variability, which potentially influences the efficacy and safety of escitalopram. Hence, it is crucial to establish a comprehensive collection of information relevant to each phenotype regarding the efficacy and tolerability of therapy. This will enable psychiatrists to make optimal decisions for individual patients.
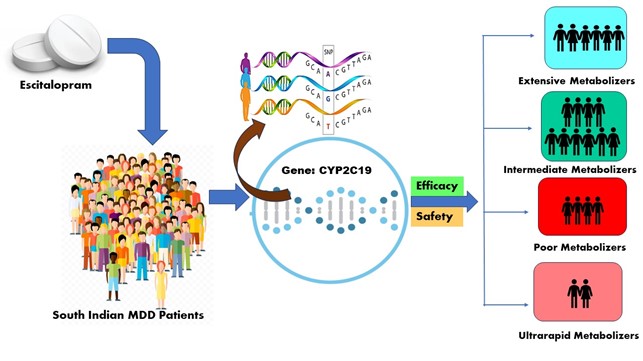
The aim of the study: The aim of this study is to classify MDD patients into various CYP2C19 metabolizer groups and to determine the association between phenotype and treatment outcome.
Materials and Methods: The study enrolled 119 escitalopram monotherapy-treated MDD patients aged 18–58. MADRS, HDRS-17, and CGI were used to measure efficacy at baseline, weeks 4, 8, and 12. Safety and tolerability outcomes were examined from occurring ADRs. Clinical outcomes were compared among phenotypes based on changes in HDRS-17 and CGI scores from week 4 to week 12.
Results: Subjects were categorized by CYP2C19 genotype: 20 poor (PM), 64 intermediate (IM), 24 extensive (EM), and 11 ultra-rapid (UM) metabolizers. Response and remission occurred in 67.2 % and 26.8 % of the 119 subjects at the end of the 12th week of the study. The response rate in PM was much lower (21.6 %) compared to EM. There were 312 adverse drug reactions (ADRs), and 88 (73.94 %) individuals had at least one. In safety data, nervousness was the most common ADR among the four groups 66 (55.4 %), followed by decreased appetite 48 (40.3 %). There were no severe ADRs. Men had more ADRs than women.
Conclusion: CYP2C19 genotyping may help personalize escitalopram medication. The study found that the reduced ability of PM to metabolize escitalopram is probably associated with the decreased efficacy and tolerance shown in PM compared to EM and IM. The relationship between metabolizer status and treatment response followed the anticipated direction. Our findings should guide future clinical studies that include pharmacokinetic assessments
References
- Sadock, B. J., Sadock, V. A., Ruiz, P. (2015). Mood disorders. Kaplan and Sadock’s synopsis of psychiatry: Behavioural sciences/Clinical psychiatry. Indian Journal of Psychiatry, 11, 347–350.
- Arvind, B. A., Gururaj, G., Loganathan, S., Amudhan, S., Varghese, M., Benegal, V. et al. (2019). Prevalence and socioeconomic impact of depressive disorders in India: multisite population-based cross-sectional study. BMJ Open, 9 (6), e027250. https://doi.org/10.1136/bmjopen-2018-027250
- Dhar, A. K., Barton, D. A. (2016). Depression and the link with cardiovascular disease. Front Psychiatry, 7. https://doi.org/10.3389/fpsyt.2016.00033
- Xin, L.-M., Chen, L., Su, Y.-A., Yang, F.-D., Wang, G., Fang, Y.-R. et al. (2018). Risk Factors for Recent Suicide Attempts in Major Depressive Disorder Patients in China: Results From a National Study. Frontiers in Psychiatry, 9. https://doi.org/10.3389/fpsyt.2018.00300
- Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study (2017). Lancet, 392 (10159), 1789–1858.
- Mathers, C. D., Loncar, D. (2006). Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med, 3 (11), 2011–2030. https://doi.org/10.1371/journal.pmed.0030442
- Maity, N., Ghosal, M. K., Gupta, A., Sil, A., Chakraborty, S., Chatterjee, S. (2014). Clinical effectiveness and safety of escitalopram and desvenlafaxine in patients of depression with anxiety: A randomized, open-label controlled trial. Indian Journal of Pharmacology, 46, 433–437. https://doi.org/10.4103/0253-7613.135959
- Uckun, Z., Baskak, B., Ozel-Kizil, E. T., Ozdemir, H., Ozguven, H. D., Suzen, H. S. (2015). The impact of CYP2C19 polymorphisms on citalopram metabolism in patients with major depressive disorder. Journal of Clinical Pharmacy and Therapeutics, 40 (6), 672–679. https://doi.org/10.1111/jcpt.12320
- Spina, E., De Leon, J. (2015). Clinical applications of CYP genotyping in psychiatry. Journal of Neural Transmission, 122 (1), 5–28. https://doi.org/10.1007/s00702-014-1300-5
- He, Q., Yuan, Z., Liu, Y., Zhang, J., Yan, H., Shen, L. et al. (2017). Correlation between cytochrome P450 2C19 genetic polymorphism and treatment response to escitalopram in panic disorder. Pharmacogenetics and Genomics, 27 (8), 279–284. https://doi.org/10.1097/fpc.0000000000000290
- Hicks, J., Bishop, J., Sangkuhl, K., Müller, D., Ji, Y., Leckband, S. et al. (2015). Clinical Pharmacogenetics Implementation Consortium (CPIC) Guideline for CYP2D6 and CYP2C19 Genotypes and Dosing of Selective Serotonin Reuptake Inhibitors. Clinical Pharmacology & Therapeutics, 98 (2), 127–134. https://doi.org/10.1002/cpt.147
- Zhou, H.-H. (2002). Genetic polymorphism of CYP2C19 in Chinese ethnic populations. International Congress Series, 1244, 51–61. https://doi.org/10.1016/s0531-5131(02)00455-7
- Horai, Y., Nakano, M., Ishizaki, T. (1989). Metoprolol and mephenytoin oxidation polymorphisms in Far Eastern Oriental subjects: Japanese versus mainland Chinese. Clinical Pharmacology and Therapeutics, 46, 198–207. https://doi.org/10.1038/clpt.1989.126
- Yu, B. N., Chen, G. L., He, N., Ouyang, D.-S., Chen, X.-P., Liu, Z.-Q. (2003). Pharmacokinetics of citalopram in relation to genetic polymorphism of CYP2C19. Drug Metabolism and Disposition, 31, 1255–1259. https://doi.org/10.1124/dmd.31.10.1255
- Rudberg, I., Mohebi, B., Hermann, M., Refsum, H., Molden, E. (2008). Impact of the ultrarapid CYP2C19*17 allele on serum concentration of escitalopram in psychiatric patients. Clinical Pharmacology & Therapeutics, 83, 322–327. https://doi.org/10.1038/sj.clpt.6100291
- Anderson, I. M. (1998). SSRIs versus tricyclic antidepressants in depressed inpatients: A meta-analysis of efficacy and tolerability. Depression and Anxiety, 7 (S1), 11–17. https://doi.org/10.1002/(sici)1520-6394(1998)7:1+<11::aid-da4>3.0.co;2-i
- Wilkinson, G. R. (2005). Drug Metabolism and Variability among Patients in Drug Response. New England Journal of Medicine, 352 (21), 2211–2221. https://doi.org/10.1056/nejmra032424
- Rosen, R. C., Lane, R. G., Menza, M. (1999). Effects of SSRIs on sexual function: A critical review. Journal of Clinical Psychopharmacology, 19, 67–85. https://doi.org/10.1097/00004714-199902000-00013
- Trivedi, M. H., Rush, A. J., Wisniewski, S. R., Nierenberg, A. A., Warden, D., Ritz, L. et al. (2006). Evaluation of Outcomes With Citalopram for Depression Using Measurement-Based Care in STAR*D: Implications for Clinical Practice. American Journal of Psychiatry, 163 (1), 28–40. https://doi.org/10.1176/appi.ajp.163.1.28
- Ng, C., Sarris, J., Singh, A., Bousman, C., Byron, K., Peh, L. H. et al. (2013). Pharmacogenetic polymorphisms and response to escitalopram and venlafaxine over 8 weeks in major depression. Human Psychopharmacology: Clinical and Experimental, 28, 516–522. https://doi.org/10.1002/hup.2340
- Hodgson, K., Tansey, K., Dernovšek, M. Z., Hauser, J., Henigsberg, N., Maier, W. et al. (2013). Genetic differences in cytochrome P450 enzymes and antidepressant treatment response. Journal of Psychopharmacology, 28 (2), 133–141. https://doi.org/10.1177/0269881113512041
- Chang, M., Tybring, G., Dahl, M.-L., Lindh, J. D. (2014). Impact of Cytochrome P450 2C19 Polymorphisms on Citalopram/Escitalopram Exposure: A Systematic Review and Meta-Analysis. Clinical Pharmacokinetics, 53 (9), 801–811. https://doi.org/10.1007/s40262-014-0162-1
- Jukić, M. M., Haslemo, T., Molden, E., Ingelman-Sundberg, M. (2018). Impact of CYP2C19 Genotype on Escitalopram Exposure and Therapeutic Failure: A Retrospective Study Based on 2,087 Patients. American Journal of Psychiatry, 175 (5), 463–470. https://doi.org/10.1176/appi.ajp.2017.17050550
- Caudle, K. E., Dunnenberger, H. M., Freimuth, R. R., Peterson, J. F., Burlison, J. D., Whirl-Carrillo, M. et al. (2017). Standardizing terms for clinical pharmacogenetic test results: consensus terms from the Clinical Pharmacogenetics Implementation Consortium (CPIC). Genetics in Medicine, 19 (2), 215–223. https://doi.org/10.1038/gim.2016.87
- Lingjærde, O., Ahlfors, U. G., Bech, P., Dencker, S. J., Elgen, K. (1987). The UKU side effect rating scale: A new comprehensive rating scale for psychotropic drugs and a cross‐sectional study of side effects in neuroleptic‐treated patients. Acta Psychiatrica Scandinavica, 76 (s334), 1–100. https://doi.org/10.1111/j.1600-0447.1987.tb10566.x
- Huezo-Diaz, P., Perroud, N., Spencer, E. P., Smith, R., Sim, S., Virding, S. et al. (2011). CYP2C19 genotype predicts steady state escitalopram concentration in GENDEP. Journal of Psychopharmacology, 26 (3), 398–407. https://doi.org/10.1177/0269881111414451
- Rudberg, I., Hermann, M., Refsum, H., Molden, E. (2008). Serum concentrations of sertraline and N-desmethyl sertraline in relation to CYP2C19 genotype in psychiatric patients. European Journal of Clinical Pharmacology, 64 (12), 1181–1188. https://doi.org/10.1007/s00228-008-0533-3
- Aynacioglu, A., Sachse, C., Bozkurt, A., Kortunay, S., Nacak, M., Schroder, T. et al. (1999). Low frequency of defective alleles of cytochrome P450 enzymes 2C19 and 2D6 in the Turkish population. Clinical Pharmacology & Therapeutics, 66 (2), 185–192. https://doi.org/10.1053/cp.1999.v66.100072001
- Pinto, C., Trivedi, J. K., Vankar, G. K., Sharma, P. S., Narasimha, V. (2007). An open-label multicentric study of the tolerability and response to escitalopram treatment in Indian patients with major depressive disorder. Journal of Indian Medical Association, 105 (7), 364–368.
- Strumila, R., Lengvenyte, A., Ambrozaityte, L., Balkeliene, D., Utkus, A., Dlugauskas, E. (2021). CYP2C19 polymorphisms are associated with severity of depression at initial evaluation and after the treatment independently of the prescribed medications: 4 weeks prospective study. Psychiatric Genetics, 31 (5), 177–185. https://doi.org/10.1097/ypg.0000000000000287
- Yin, O. Q., Wing, Y.-K., Cheung, Y., Wang, Z.-J., Lam, S.-L., Chiu, H. F., Chow, M. S. (2006). Phenotype-genotype Relationship and Clinical Effects of Citalopram in Chinese Patients. Journal of Clinical Psychopharmacology, 26 (4), 367–372. https://doi.org/10.1097/01.jcp.0000227355.54074.14
- Fabbri, C., Tansey, K. E., Perlis, R. H., Hauser, J., Henigsberg, N., Maier, W. et al. (2018). Effect of cytochrome CYP2C19 metabolizing activity on antidepressant response and side effects: mMeta-analysis of data from genome-wide association studies. European Neuropsychopharmacology, 28 (8), 945–954. https://doi.org/10.1016/j.euroneuro.2018.05.009
- Goethe, J. W., Woolley, S. B., Cardoni, A. A., Woznicki, B. A., Piez, D. A. (2007). Selective Serotonin Reuptake Inhibitor Discontinuation: side effects and other factors that influence medication adherenc. Journal of Clinical Psychopharmacology, 27 (5), 451–458. https://doi.org/10.1097/jcp.0b013e31815152a5
- Tsai, M. H., Lin, K. M., Hsiao, M. C., Shen, W. W., Lu, M. L., Tang, H. S. et al. (2010). Genetic polymorphisms of cytochrome P450 enzymes influence metabolism of the antidepressant escitalopram and treatment response. Pharmacogenomics, 11 (4), 537–546. https://doi.org/10.2217/pgs.09.168
- Singh, A. B., Bousman, C. A., Ng, C. H., Byron, K., Berk, M. (2012). ABCB1 polymorphism predicts escitalopram dose needed for remission in major depression. Translational Psychiatry, 27 (2), e198. https://doi.org/10.1038/tp.2012.115
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2024 B Jeevan Kumar, Vijayakumar Thangavel Mahalingam, Ganesh Kumar

This work is licensed under a Creative Commons Attribution 4.0 International License.
Our journal abides by the Creative Commons CC BY copyright rights and permissions for open access journals.







