Study of the potential antipsoriatic effectiveness and safety of the combination of naftalan oil with salicylic acid in cellular test systems
DOI:
https://doi.org/10.15587/2519-4852.2025.324050Keywords:
purified naftalan oil, salicylic acid, betamethasone dipropionate, psoriasis, human keratinocytes, cell viability, apoptosis, interleukinsAbstract
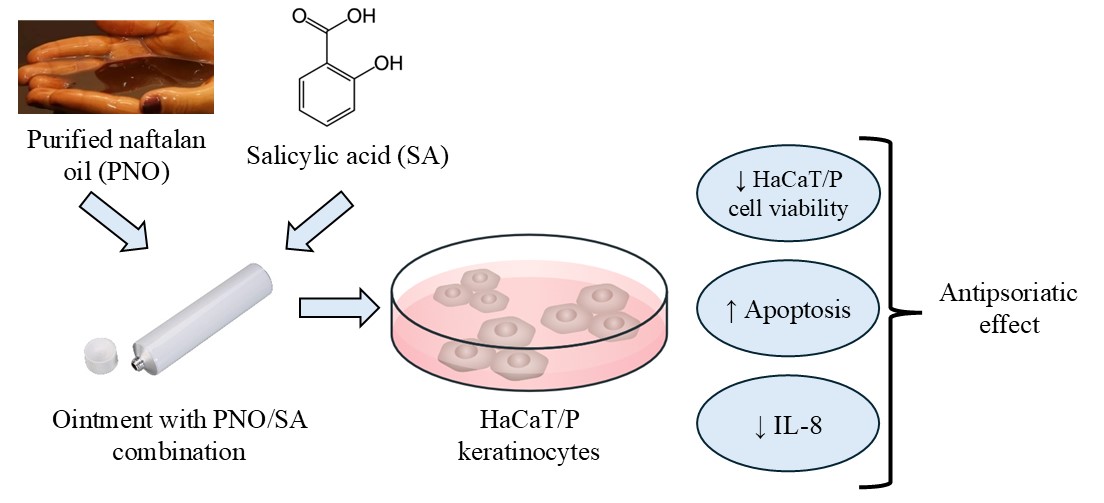
The aim of this work was to evaluate the combination of purified naftalan oil (PNO) with salicylic acid (SA) for topical application as a potential effective antipsoriatic ointment and to study the mechanism of its action using specific in vitro cell models.
Material and methods. The effectiveness and safety of the following dosage forms were studied: ointment with 10 % PNO and 3 % SA (PNO-SA), cream with 0.064 % betamethasone dipropionate (BD), and ointment with 3 % SA. Cell viability of original HaCaT human keratinocytes and modified HaCaT/P was determined by colorimetric methods, namely by crystal violet staining or the MTT test method. The level of apoptosis of cells was evaluated by flow cytometry. Production of pro-inflammatory cytokines IL-8 and IL-1β was measured by the ELISA method.
Results. It was shown that the sensitivity of cells with a psoriasis-like phenotype (HaCaT/P) to PNO increases statistically significantly compared to control cells, which was confirmed by both the cell viability and the MTT test. to obtain a result with inhibition of cells with psoriasis-like characteristics, HaCaT/P requires a smaller concentration of the drug compared to a similar effect on conditionally normal cells – HaCaT. This may indicate the relative safety of the proposed medicinal product (combination) in parallel with the conditions of its effectiveness concerning pathologically changed cells. The results of flow cytometry showed that the new PNO-SA complex causes a statistically significant increase in the percentage of cells in all phases of apoptosis compared to control cells. Finally, a statistically significant decrease in the production of IL-8 by cells with psoriasis-like characteristics – the HaCaT/P line was shown in the presence of the PNO-SA combination compared with control cells. In addition, there was a significant decrease in the level of IL-8 production in cells in the presence of the combination compared to SA and the comparator BD. However, in terms of its effect on IL-1β production, the PNO-SA combination proved to be inactive.
Conclusion. Our proposed combination (PNO-SA), which suppresses pro-inflammatory IL-8 by more than 2 times (by 67.4 %) compared to the control, is a promising potential option for local treatment of psoriatic lesions. We speculate about the future demand of this combination in the clinical setting because along with high efficiency (in terms of viability indicators – the number of living cells, the level of apoptosis) towards pathologically changed keratinocytes, it shows a low toxicity profile towards normal healthy keratinocytes
References
- Sarama, R., Matharu, P. K., Abduldaiem, Y., Corrêa, M. P., Gil, C. D., Greco, K. V. (2022). In Vitro Disease Models for Understanding Psoriasis and Atopic Dermatitis. Frontiers in Bioengineering and Biotechnology, 10. https://doi.org/10.3389/fbioe.2022.803218
- Michalek, I. M., Loring, B., John, S. M. (2016). A systematic review of worldwide epidemiology of psoriasis. Journal of the European Academy of Dermatology and Venereology, 31 (2), 205–212. https://doi.org/10.1111/jdv.13854
- Rendon, A., Schäkel, K. (2019). Psoriasis Pathogenesis and Treatment. International Journal of Molecular Sciences, 20 (6), 1475. https://doi.org/10.3390/ijms20061475
- Zhou, X., Chen, Y., Cui, L., Shi, Y., Guo, C. (2022). Advances in the pathogenesis of psoriasis: from keratinocyte perspective. Cell Death & Disease, 13 (1). https://doi.org/10.1038/s41419-022-04523-3
- Cai, Y., Xue, F., Quan, C., Qu, M., Liu, N., Zhang, Y. et al. (2019). A Critical Role of the IL-1β–IL-1R Signaling Pathway in Skin Inflammation and Psoriasis Pathogenesis. Journal of Investigative Dermatology, 139 (1), 146–156. https://doi.org/10.1016/j.jid.2018.07.025
- Reid, C., Griffiths, C. (2020). Psoriasis and Treatment: Past, Present and Future Aspects. Acta Dermato Venereologica, 100 (3), 70–80. https://doi.org/10.2340/00015555-3386
- Ramic, L., Sator, P. (2023). Topical treatment of psoriasis vulgaris. JDDG: Journal Der Deutschen Dermatologischen Gesellschaft, 21 (6), 631–642. Portico. https://doi.org/10.1111/ddg.15042;
- Gisondi, P., Gracia-Cazaña, T., Kurzen, H., Galván, J. (2024). Calcipotriol/Betamethasone Dipropionate for the Treatment of Psoriasis: Mechanism of Action and Evidence of Efficacy and Safety versus Topical Corticosteroids. Journal of Clinical Medicine, 13 (15), 4484. https://doi.org/10.3390/jcm13154484
- Lee, H.-J., Kim, M. (2023). Challenges and Future Trends in the Treatment of Psoriasis. International Journal of Molecular Sciences, 24 (17), 13313. https://doi.org/10.3390/ijms241713313
- Schlager, J. G., Rosumeck, S., Werner, R. N., Jacobs, A., Schmitt, J., Schlager, C., Nast, A. (2016). Topical treatments for scalp psoriasis. Cochrane Database of Systematic Reviews, 2016 (2). https://doi.org/10.1002/14651858.cd009687.pub2
- Adigozalova, V. A. (2023). Naftalan oil is a specific balneological factor of Azerbaijan. Azerbaijan Journal of Physiology, 1, 48–56. https://doi.org/10.59883/ajp.52
- Flutter, B., Nestle, F. O. (2013). TLRs to cytokines: Mechanistic insights from the imiquimod mouse model of psoriasis. European Journal of Immunology, 43 (12), 3138–3146. https://doi.org/10.1002/eji.201343801
- Strychalski, M. L., Brown, H. S., Bishop, S. C. (2022). Cytokine Modulators in Plaque Psoriasis – A Review of Current and Prospective Biologic Therapeutic Approaches. JAAD International, 9, 82–91. https://doi.org/10.1016/j.jdin.2022.08.008
- Dopytalska, K., Ciechanowicz, P., Wiszniewski, K., Szymańska, E., Walecka, I. (2021). The Role of Epigenetic Factors in Psoriasis. International Journal of Molecular Sciences, 22 (17), 9294. https://doi.org/10.3390/ijms22179294
- Draelos, Z. D. (2022). The Efficacy and Tolerability of Turmeric and Salicylic Acid in Psoriasis Treatment. Psoriasis: Targets and Therapy, 12, 63–71. https://doi.org/10.2147/ptt.s360448
- Elmets, C. A., Korman, N. J., Prater, E. F., Wong, E. B., Rupani, R. N., Kivelevitch, D. et al. (2021). Joint AAD–NPF Guidelines of care for the management and treatment of psoriasis with topical therapy and alternative medicine modalities for psoriasis severity measures. Journal of the American Academy of Dermatology, 84 (2), 432–470. https://doi.org/10.1016/j.jaad.2020.07.087
- Krnjevic-Pezic, G., Alajbeg, I., Maricić, G., Pašić, A., Ćurković, B., Ceović, R., Kostović, K. (2012). Naphthalan Oil in the Treatment of Psoriasis and Psoriatic Arthritis. Psoriasis Forum, 18a (1), 26–31. https://doi.org/10.1177/247553031218a00104
- Krawczyk, A., Miśkiewicz, J., Strzelec, K., Wcisło-Dziadecka, D., Strzalka-Mrozik, B. (2020). Apoptosis in Autoimmunological Diseases, with Particular Consideration of Molecular Aspects of Psoriasis. Medical Science Monitor, 26. https://doi.org/10.12659/msm.922035
- Tsai, Y.-C., Tsai, T.-F. (2017). Anti-interleukin and interleukin therapies for psoriasis: current evidence and clinical usefulness. Therapeutic Advances in Musculoskeletal Disease, 9 (11), 277–294. https://doi.org/10.1177/1759720x17735756
- Duan, H., Koga, T., Kohda, F., Hara, H., Urabe, K., Furue, M. (2001). Interleukin-8-positive neutrophils in psoriasis. Journal of Dermatological Science, 26(2), 119–124. https://doi.org/10.1016/s0923-1811(00)00167-5
- Huang, Z. (2002). En bo ke zhi liao yin xie bing de xinji yuan. China Prescription Drug, 5, 54–56.
- Krueger, G. G. (2001). ABX-IL 8 in the treatment of psoriasis: clinical results. 2nd joint meeting international psoriasis symposium and European congress on psoriasis. San Francisco, 122.
- García-Martín, E., Romero-Jiménez, R., Baniandrés-Rodríguez, O., Escudero-Vilaplana, V., Benedí-González, J., de los Ríos Luna, P. M. et al. (2023). Anti-interleukin-17 therapies for moderate/severe psoriasis in clinical practice: effectiveness, safety and association with clinical patient factors. European Journal of Hospital Pharmacy, 31 (5), 409–415. https://doi.org/10.1136/ejhpharm-2022-003594
- Graier, T., Weger, W., Jonak, C., Sator, P., Zikeli, C., Prillinger, K. et al. (2022). Real-world effectiveness of anti-interleukin-23 antibodies in chronic plaque-type psoriasis of patients from the Austrian Psoriasis Registry (PsoRA). Scientific Reports, 12 (1). https://doi.org/10.1038/s41598-022-18790-9
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2025 Ganna Zaychenko, Iryna Stan, Pavlo Simonov, Oksana Sinitsyna, Oksana Simonova

This work is licensed under a Creative Commons Attribution 4.0 International License.
Our journal abides by the Creative Commons CC BY copyright rights and permissions for open access journals.






