Похідні на основі тіазолідинону як терапевтичні агенти подвійної дії: антимікробна ефективність, цитотоксичність та фармакокінетичний потенціал
DOI:
https://doi.org/10.15587/2519-4852.2025.342467Ключові слова:
протимікробна активність, цитотоксичність, фармакокінетика, молекулярний докінг, молекулярна динаміка, тіазолідинон, ProTox IIАнотація
Інфекційні захворювання та рак залишаються провідними глобальними викликами для системи охорони здоров’я, при цьому зростає резистентність до наявних антибіотиків і спостерігається обмежена селективність багатьох цитотоксичних агентів. Гетероциклічні каркаси, зокрема тіазолідони, становлять перспективну платформу для створення нових протимікробних та протипухлинних сполук.
Мета дослідження. Оцінити протимікробні та цитотоксичні властивості сполук на основі 4-тіазолідинону щодо панелі патогенних мікроорганізмів і ліній ракових клітин людини, а також визначити найбільш перспективні похідні з сприятливими показниками безпеки, фармакокінетики та механістичними характеристиками за результатами молекулярного докінгу та молекулярної динаміки.
Матеріали та методи. Бібліотека похідних 5-енамін(гідразин)-4-тіазолідинону була протестована на протимікробну активність проти грампозитивних і грамнегативних бактерій та Candida albicans, а також на цитотоксичність щодо шести ліній ракових клітин людини. Для вибраних сполук визначали мінімальні інгібуючі концентрації (MIC) та значення IC₅₀. Фармакокінетичні властивості, включаючи шлунково-кишкову абсорбцію та ліпофільність, оцінювали in silico. Для дослідження можливих механізмів антибактеріальної дії проводили молекулярний докінг до MurB (UDP-N-ацетиленолпірувілглюкозамінредуктази) та субодиниці B ДНК-гірази (АТФазний домен), після чого проводили дослідження методом молекулярної динаміки (MD) для оцінки стабільності найбільш перспективних комплексів.
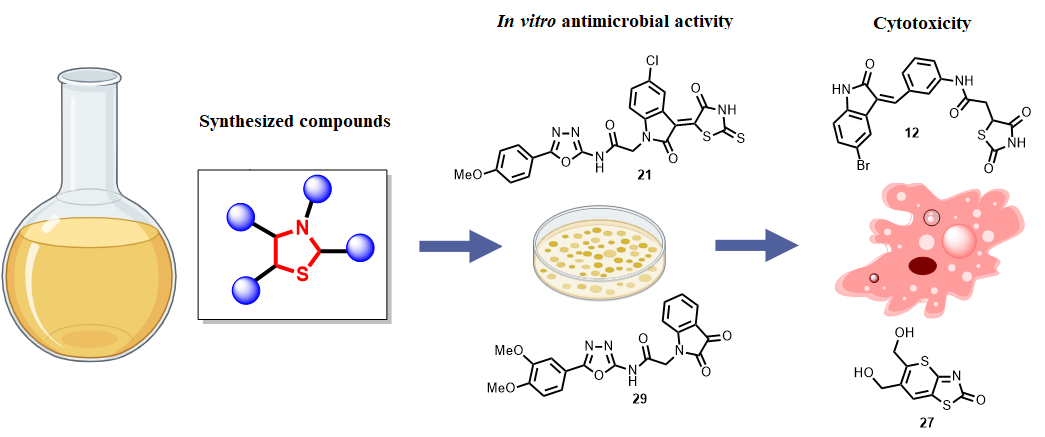
Результати. Тридцять дві сполуки проявили протимікробну активність (MIC ≤ 200 мкМ), і десять з них (6, 7, 10, 12, 13, 16, 19, 21, 22 та 29) були визначені як найбільш активні. Сполука 29, гібрид ізатину з оксадіазолом, продемонструвала потужну активність проти Enterococcus faecalis та ванкоміцин-резистентного E. faecium (MIC = 3,13 мкМ), перевищуючи ефективність ванкоміцину. Сполука 21 виявила високу активність проти Staphylococcus epidermidis (MIC = 1,56 мкМ), тоді як сполука 6 була ефективною проти метицилін-чутливого та метицилін-резистентного S. aureus (MIC = 6,25 мкМ). Помірну протигрибкову активність виявлено для сполуки 27 (MIC = 100 мкМ), тоді як грамнегативні бактерії здебільшого залишалися резистентними. Скринінг цитотоксичності показав вибіркову протиракову активність сполук 12 та 27 із високим терапевтичним індексом щодо клітин CCRF-CEM та мінімальним впливом на нормальні фібробласти. Сполука 2 проявила сильну цитотоксичність (IC₅₀ = 1,1 мкМ), тоді як сполука 29 поєднувала відсутність цитотоксичності з сприятливими фармакокінетичними характеристиками.
Молекулярний докінг підтвердив MurB як основну антибактеріальну мішень: найбільш активні сполуки (21 і 29) мали найвигідніші енергії зв’язування. Сполука 29 також продемонструвала високу спорідненість до GyrB, що вказує на можливий механізм їх подвійної дії. Результати молекулярно-динамічного моделювання засвідчили високу стабільність комплексів MurB–сполука 29, що особливо добре узгоджується з експериментально визначеною антибактеріальною активністю.
Висновки. Гібридні сполуки на основі тіазолідинону продемонстрували перспективні протимікробні та протиракові властивості. Серед них сполука 29 вирізняється як особливо перспективний кандидат із подвійною фармакологічною дією завдяки високій активності, сприятливому профілю безпеки, оптимальним фармакокінетичним характеристикам та підтвердженій взаємодії з ключовими бактеріальними ферментами. Сукупність біологічних і комп’ютерних даних підтверджує потенціал тіазолідинонового скелета як основи для створення селективних або мультитаргетних терапевтичних агентів
Спонсори дослідження
- National Research Foundation of Ukraine under project number 2023.05/0021
- Scientific (research and development) project under number 0125U003375
- Projects IGA_LF_2024_034 and IGA_LF_2024_038
- National Institute of Virology and Bacteriology project (Program EXCELES, ID Project No. LX22NPO5103)
- European Union–Next Generation EU
Посилання
- Singh, K. S., Anand, S., Dholpuria, S., Sharma, J. K., Blankenfeldt, W., Shouche, Y. (2021). Antimicrobial resistance dynamics and the one-health strategy: a review. Environmental Chemistry Letters, 19 (4), 2995–3007. https://doi.org/10.1007/s10311-021-01238-3
- Roy, S., Sarkhel, S., Bisht, D., Hanumantharao, S. N., Rao, S., Jaiswal, A. (2022). Antimicrobial mechanisms of biomaterials: from macro to nano. Biomaterials Science, 10 (16), 4392–4423. https://doi.org/10.1039/d2bm00472k
- Church, N. A., McKillip, J. L. (2021). Antibiotic resistance crisis: challenges and imperatives. Biologia, 76 (5), 1535–1550. https://doi.org/10.1007/s11756-021-00697-x
- Grant, E. B., Guiadeen, D., Baum, E. Z., Foleno, B. D., Jin, H., Montenegro, D. A. et al. (2000). The synthesis and SAR of rhodanines as novel class C β-lactamase inhibitors. Bioorganic & Medicinal Chemistry Letters, 10 (19), 2179–2182. https://doi.org/10.1016/s0960-894x(00)00444-3
- Suree, N., Yi, S. W., Thieu, W., Marohn, M., Damoiseaux, R., Chan, A. et al. (2009). Discovery and structure–activity relationship analysis of Staphylococcus aureus sortase A inhibitors. Bioorganic & Medicinal Chemistry, 17 (20), 7174–7185. https://doi.org/10.1016/j.bmc.2009.08.067
- Howard, M. H., Cenizal, T., Gutteridge, S., Hanna, W. S., Tao, Y., Totrov, M. et al. (2004). A Novel Class of Inhibitors of Peptide Deformylase Discovered through High-Throughput Screening and Virtual Ligand Screening. Journal of Medicinal Chemistry, 47 (27), 6669–6672. https://doi.org/10.1021/jm049222o
- Carlson, E. E., May, J. F., Kiessling, L. L. (2006). Chemical Probes of UDP-Galactopyranose Mutase. Chemistry & Biology, 13 (8), 825–837. https://doi.org/10.1016/j.chembiol.2006.06.007
- Sim, M. M., Ng, S. B., Buss, A. D., Crasta, S. C., Goh, K. L., Lee, S. K. (2002). Benzylidene Rhodanines as Novel Inhibitors of UDP-N-Acetylmuramate/l-Alanine Ligase. Bioorganic & Medicinal Chemistry Letters, 12 (4), 697–699. https://doi.org/10.1016/s0960-894x(01)00832-0
- Smith, T. K., Young, B. L., Denton, H., Hughes, D. L., Wagner, G. K. (2009). First small molecular inhibitors of T. brucei dolicholphosphate mannose synthase (DPMS), a validated drug target in African sleeping sickness. Bioorganic & Medicinal Chemistry Letters, 19 (6), 1749–1752. https://doi.org/10.1016/j.bmcl.2009.01.083
- Vicini, P., Geronikaki, A., Anastasia, K., Incerti, M., Zani, F. (2006). Synthesis and antimicrobial activity of novel 2-thiazolylimino-5-arylidene-4-thiazolidinones. Bioorganic & Medicinal Chemistry, 14 (11), 3859–3864. https://doi.org/10.1016/j.bmc.2006.01.043
- Horishny, V., Kartsev, V., Geronikaki, A., Matiychuk, V., Petrou, A., Glamoclija, J. et al. (2020). 5-(1H-Indol-3-ylmethylene)-4-oxo-2-thioxothiazolidin-3-yl)alkancarboxylic Acids as Antimicrobial Agents: Synthesis, Biological Evaluation, and Molecular Docking Studies. Molecules, 25 (8), 1964. https://doi.org/10.3390/molecules25081964
- Soltero-Higgin, M., Carlson, E. E., Phillips, J. H., Kiessling, L. L. (2004). Identification of Inhibitors for UDP-Galactopyranose Mutase. Journal of the American Chemical Society, 126 (34), 10532–10533. https://doi.org/10.1021/ja048017v
- Lozynskyi, A., Zasidko, V., Atamanyuk, D., Kaminskyy, D., Derkach, H., Karpenko, O. et al. (2017). Synthesis, antioxidant and antimicrobial activities of novel thiopyrano[2,3-d]thiazoles based on aroylacrylic acids. Molecular Diversity, 21 (2), 427–436. https://doi.org/10.1007/s11030-017-9737-8
- Habib, N. S., Rida, S. M., Badawey, E. A. M., Fahmy, H. T. Y. (1996). Condensed thiazoles, I: Synthesis of 5,7-disubstituted thiazolo[4,5-d]pyrimidines as possible anti-HIV, anticancer, and antimicrobial agents. Monatshefte für Chemie Chemical Monthly, 127 (11), 1203–1207. https://doi.org/10.1007/bf00844696
- Mahfouz, Aziz, A. A., Elhabashy, F. M. (1990). New synthesis of 2-substituted imidazo[2,1-b]thiazoles and their antimicrobial activities. Archives of Pharmacal Research, 13 (1), 9–13. https://doi.org/10.1007/bf02857826
- Lozynskyi, A., Konechnyi, Y., Senkiv, J., Yushyn, I., Khyluk, D., Karpenko, O. et al. (2021). Synthesis and Biological Activity Evaluation of Novel 5-Methyl-7-phenyl-3H-thiazolo[4,5-b]pyridin-2-ones. Scientia Pharmaceutica, 89 (4), 52. https://doi.org/10.3390/scipharm89040052
- Khamitova, А., Berillo, D., Lozynskyi, A., Konechnyi, Y., Mural, D., Georgiyants, V., Lesyk, R. (2024). Thiadiazole and Thiazole Derivatives as Potential Antimicrobial Agents. Mini-Reviews in Medicinal Chemistry, 24 (5), 531–545. https://doi.org/10.2174/1389557523666230713115947
- Currie, G. M. (2018). Pharmacology, Part 1: Introduction to Pharmacology and Pharmacodynamics. Journal of Nuclear Medicine Technology, 46 (2), 81–86. https://doi.org/10.2967/jnmt.117.199588
- Shepeta, Y., Lozynskyi, A., Sulyma, M., Nektegayev, I., Grellier, P., Lesyk, R. (2020). Synthesis and biological activity evaluation of new thiazolidinone-diclofenac hybrid molecules. Phosphorus, Sulfur, and Silicon and the Related Elements, 195 (10), 836–841. https://doi.org/10.1080/10426507.2020.1759060
- Kryshchyshyn, A., Kaminskyy, D., Karpenko, O., Gzella, A., Grellier, P., Lesyk, R. (2019). Thiazolidinone/thiazole based hybrids – New class of antitrypanosomal agents. European Journal of Medicinal Chemistry, 174, 292–308. https://doi.org/10.1016/j.ejmech.2019.04.052
- Sklyarova, Y., Fomenko, I., Lozynska, I., Lozynskyi, A., Lesyk, R., Sklyarov, A. (2017). Hydrogen Sulfide Releasing 2-Mercaptoacrylic Acid-Based Derivative Possesses Cytoprotective Activity in a Small Intestine of Rats with Medication-Induced Enteropathy. Scientia Pharmaceutica, 85 (4), 35. https://doi.org/10.3390/scipharm85040035
- Holota, S., Kryshchyshyn, A., Derkach, H., Trufin, Y., Demchuk, I., Gzella, A. et al. (2019). Synthesis of 5-enamine-4-thiazolidinone derivatives with trypanocidal and anticancer activity. Bioorganic Chemistry, 86, 126–136. https://doi.org/10.1016/j.bioorg.2019.01.045
- Zelisko, N., Atamanyuk, D., Ostapiuk, Y., Bryhas, A., Matiychuk, V., Gzella, A., Lesyk, R. (2015). Synthesis of fused thiopyrano[2,3-d][1,3]thiazoles via hetero-Diels–Alder reaction related tandem and domino processes. Tetrahedron, 71 (50), 9501–9508. https://doi.org/10.1016/j.tet.2015.10.019
- Kryshchyshyn, A., Kaminskyy, D., Nektegayev, I., Grellier, P., Lesyk, R. (2018). Isothiochromenothiazoles – A Class of Fused Thiazolidinone Derivatives with Established Anticancer Activity That Inhibits Growth of Trypanosoma brucei brucei. Scientia Pharmaceutica, 86 (4), 47. https://doi.org/10.3390/scipharm86040047
- Lozynskyi, A., Zimenkovsky, B., Lesyk, R. (2014). Synthesis and Anticancer Activity of New Thiopyrano[2,3-d]thiazoles Based on Cinnamic Acid Amides. Scientia Pharmaceutica, 82 (4), 723–733. https://doi.org/10.3797/scipharm.1408-05
- Konechnyi, Y. T., Lozynskyi, A. V., Horishny, V. Ya., Konechna, R. T., Vynnytska, R. B., Korniychuk, O. P., Lesyk, R. B. (2020). Synthesis of indoline-thiazolidinone hybrids with antibacterial and antifungal activities. Biopolymers and Cell, 36 (5), 381–391. https://doi.org/10.7124/bc.000a3a
- SwissADME online server of the Swiss Institute of Bioinformatics. Available at: http://www.swissadme.ch/index.php
- Banerjee, P., Eckert, A. O., Schrey, A. K., Preissner, R. (2018). ProTox-II: a webserver for the prediction of toxicity of chemicals. Nucleic Acids Research, 46 (W1), W257–W263. https://doi.org/10.1093/nar/gky318
- Kamiloglu, S., Sari, G., Ozdal, T., Capanoglu, E. (2020). Guidelines for cell viability assays. Food Frontiers, 1 (3), 332–349. https://doi.org/10.1002/fft2.44
- Markossian, S., Coussens, N. P., Dahlin, J. L., Sitta Sittampalam, G. (2021). Assay Guidance Manual for Drug Discovery: Technologies That Matter. SLAS Technology, 26 (6), 553–554. https://doi.org/10.1177/24726303211056338
- Borková, L., Frydrych, I., Jakubcová, N., Adámek, R., Lišková, B., Gurská, S. et al. (2020). Synthesis and biological evaluation of triterpenoid thiazoles derived from betulonic acid, dihydrobetulonic acid, and ursonic acid. European Journal of Medicinal Chemistry, 185, 111806. https://doi.org/10.1016/j.ejmech.2019.111806
- Jurášek, M., Řehulka, J., Hrubá, L., Ivanová, A., Gurská, S., Mokshyna, O. et al. (2023). Triazole-based estradiol dimers prepared via CuAAC from 17α-ethinyl estradiol with five-atom linkers causing G2/M arrest and tubulin inhibition. Bioorganic Chemistry, 131, 106334. https://doi.org/10.1016/j.bioorg.2022.106334
- EUCAST: Clinical breakpoints and dosing of antibiotics (2024). European Committee on Antimicrobial Susceptibility Testing, & European Committee on Antimicrobial Susceptibility Testing.
- Andres, C. J., Bronson, J. J., D’Andrea, S. V., Deshpande, M. S., Falk, P. J., Grant-Young, K. A. et al. (2000). 4-Thiazolidinones: novel inhibitors of the bacterial enzyme murB. Bioorganic & Medicinal Chemistry Letters, 10 (8), 715–717. https://doi.org/10.1016/s0960-894x(00)00073-1
- Ahmed, S., Zayed, M., El-Messery, S., Al-Agamy, M., Abdel-Rahman, H. (2016). Design, Synthesis, Antimicrobial Evaluation and Molecular Modeling Study of 1,2,4-Triazole-Based 4-Thiazolidinones. Molecules, 21 (5), 568. https://doi.org/10.3390/molecules21050568
- Pitta, E., Tsolaki, E., Geronikaki, A., Petrović, J., Glamočlija, J., Soković, M. et al. (2015). 4-Thiazolidinone derivatives as potent antimicrobial agents: microwave-assisted synthesis, biological evaluation and docking studies. MedChemComm, 6 (2), 319–326. https://doi.org/10.1039/c4md00399c
- Jakopin, Ž., Ilaš, J., Barančoková, M., Brvar, M., Tammela, P., Sollner Dolenc, M. et al. (2017). Discovery of substituted oxadiazoles as a novel scaffold for DNA gyrase inhibitors. European Journal of Medicinal Chemistry, 130, 171–184. https://doi.org/10.1016/j.ejmech.2017.02.046
- Song, F., Li, Z., Bian, Y., Huo, X., Fang, J., Shao, L., Zhou, M. (2020). Indole/isatin‐containing hybrids as potential antibacterial agents. Archiv Der Pharmazie, 353 (10). https://doi.org/10.1002/ardp.202000143
- Yang, Y.-S., Su, M.-M., Xu, J.-F., Liu, Q.-X., Bai, L.-F., Hu, X.-W., Zhu, H.-L. (2019). Discovery of novel oxoindolin derivatives as atypical dual inhibitors for DNA Gyrase and FabH. Bioorganic Chemistry, 93, 103309. https://doi.org/10.1016/j.bioorg.2019.103309
- Benson, T. E., Harris, M. S., Choi, G. H., Cialdella, J. I., Herberg, J. T., Martin, J. P., Baldwin, E. T. (2001). A Structural Variation for MurB: X-ray Crystal Structure of Staphylococcus aureus UDP-N-Acetylenolpyruvylglucosamine Reductase (MurB). Biochemistry, 40 (8), 2340–2350. https://doi.org/10.1021/bi002162d
- Ronkin, S. M., Badia, M., Bellon, S., Grillot, A.-L., Gross, C. H., Grossman, T. H. et al. (2010). Discovery of pyrazolthiazoles as novel and potent inhibitors of bacterial gyrase. Bioorganic & Medicinal Chemistry Letters, 20 (9), 2828–2831. https://doi.org/10.1016/j.bmcl.2010.03.052
- Bronson, J. J., DenBleyker, K. L., Falk, P. J., Mate, R. A., Ho, H.-T., Pucci, M. J., Snyder, L. B. (2003). Discovery of the first antibacterial small molecule inhibitors of MurB. Bioorganic & Medicinal Chemistry Letters, 13 (5), 873–875. https://doi.org/10.1016/s0960-894x(02)01076-4
- Hanwell, M. D., Curtis, D. E., Lonie, D. C., Vandermeersch, T., Zurek, E., Hutchison, G. R. (2012). Avogadro: an advanced semantic chemical editor, visualization, and analysis platform. Journal of Cheminformatics, 4 (1). https://doi.org/10.1186/1758-2946-4-17
- Halgren, T. A. (1999). MMFF VII. Characterization of MMFF94, MMFF94s, and other widely available force fields for conformational energies and for intermolecular-interaction energies and geometries. Journal of Computational Chemistry, 20 (7), 730–748. https://doi.org/10.1002/(sici)1096-987x(199905)20:7<730::aid-jcc8>3.0.co;2-t
- Morris, G. M., Huey, R., Olson, A. J. (2008). Using AutoDock for Ligand‐Receptor Docking. Current Protocols in Bioinformatics, 24 (1). https://doi.org/10.1002/0471250953.bi0814s24
- Eberhardt, J., Santos-Martins, D., Tillack, A. F., Forli, S. (2021). AutoDock Vina 1.2.0: New Docking Methods, Expanded Force Field, and Python Bindings. Journal of Chemical Information and Modeling, 61 (8), 3891–3898. https://doi.org/10.1021/acs.jcim.1c00203
- Agarwal, R., Smith, J. C. (2023). Speed vs Accuracy: Effect on Ligand Pose Accuracy of Varying Box Size and Exhaustiveness in AutoDock Vina. Molecular Informatics, 42 (2). https://doi.org/10.1002/minf.202200188
- Van Der Spoel, D., Lindahl, E., Hess, B., Groenhof, G., Mark, A. E., Berendsen, H. J. C. (2005). GROMACS: Fast, flexible, and free. Journal of Computational Chemistry, 26 (16), 1701–1718. https://doi.org/10.1002/jcc.20291
- Wang, J., Wolf, R. M., Caldwell, J. W., Kollman, P. A., Case, D. A. (2004). Development and testing of a general amber force field. Journal of Computational Chemistry, 25 (9), 1157–1174. https://doi.org/10.1002/jcc.20035
- Guex, N., Peitsch, M. C. (1997). SWISS‐MODEL and the Swiss‐Pdb Viewer: An environment for comparative protein modeling. Electrophoresis, 18 (15), 2714–2723. https://doi.org/10.1002/elps.1150181505
- Rekha, S. R., Kulandhaivel, M., Hridhya, K. V. (2018). Antibacterial Efficacy and Minimum Inhibitory Concentrations of Medicinal Plants Against Wound Pathogens. Biomedical and Pharmacology Journal, 11 (1), 237–246. https://doi.org/10.13005/bpj/1368
- Tangadanchu, V. K. R., Sui, Y.-F., Zhou, C.-H. (2021). Isatin-derived azoles as new potential antimicrobial agents: Design, synthesis and biological evaluation. Bioorganic & Medicinal Chemistry Letters, 41, 128030. https://doi.org/10.1016/j.bmcl.2021.128030
- Cheerala, V. S. K., Akhir, A., Saxena, D., Maitra, R., Chopra, S., Neelakantan, S. C. (2023). Discovery of benzoxazole–thiazolidinone hybrids as promising antibacterial agents against Staphylococcus aureus and Enterococcus species. RSC Medicinal Chemistry, 14 (9), 1712–1721. https://doi.org/10.1039/d3md00290j
##submission.downloads##
Опубліковано
Як цитувати
Номер
Розділ
Ліцензія
Авторське право (c) 2025 Dmytro Mural, Dmytro Khyluk, Andrii Lozynskyi, Victoriya Georgiyants, Olexandra Roman, Anna Kryshchyshyn-Dylevych, Sona Gurska, Pavel Polishchuk, Petr Dzubak, Marian Hajduch, Katerina Bogdanova, Kristyna Resova, Milan Kolar, Roman Lesyk

Ця робота ліцензується відповідно до Creative Commons Attribution 4.0 International License.
Наше видання використовує положення про авторські права Creative Commons CC BY для журналів відкритого доступу.








