Production of physiologically complete drinking water using modified reverse osmosis membrane elements
DOI:
https://doi.org/10.15587/1729-4061.2023.277491Keywords:
reverse osmosis, modified membrane elements, predefined selectivity, physiologically complete waterAbstract
Drinking water prepared using the most effective and popular reverse osmosis method is absolutely safe but for the most part does not meet the requirements for physiologically complete water. The latter must meet, in addition to the basic requirements, the following requirements: salt content, at least 100, and not more than 1000 mg/dm3; total hardness; in the range of 1–7.0 mmol/dm3. Now, to fulfill these requirements, the stage after desalting employs various methods of domineralization of reverse osmosis water, each of which has certain disadvantages.
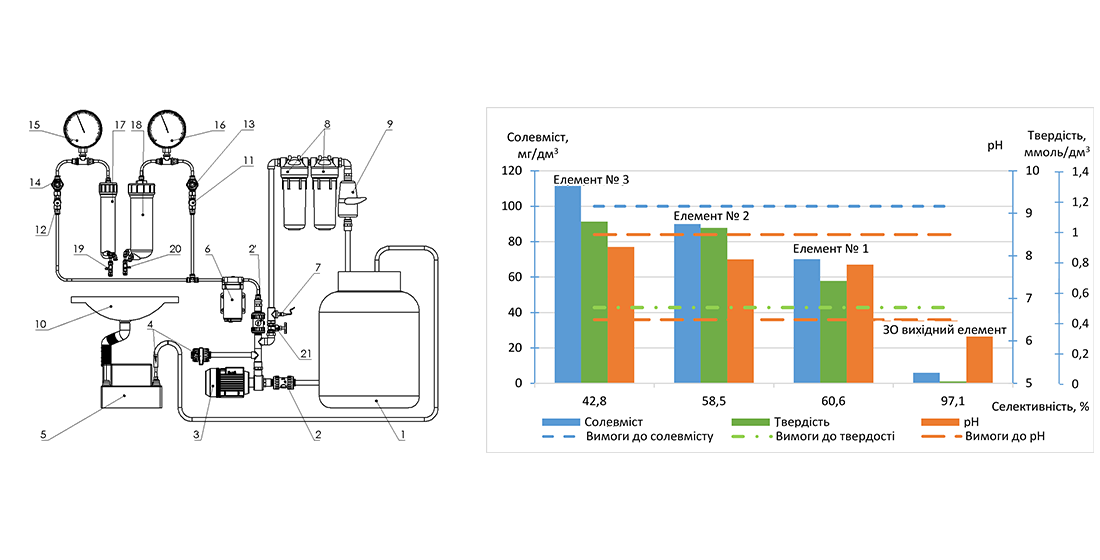
This paper considers the task of obtaining safe physiologically complete water immediately after the stage of membrane desalting by using modified reverse osmosis membrane elements with the predefined selectivity. The study object was the process of obtaining reverse osmosis membrane elements with the predefined selectivity by modifying them with sodium hypochlorite solution for use in the process of obtaining physiologically complete drinking water.
The required level of selectivity of modified elements was calculated to obtain safe physiologically complete water from starting water, depending on its salt content. Thus, for the starting water with a salt content of 200–300 mg/dm3, the specified selectivity of the membrane element should be no more than 60 % at a temperature of 25 °C. Rational conditions for conducting the modification process for obtaining a membrane element with such exact selectivity have been established. The nature of the influence of changes in water temperature on the selectivity of the modified element was studied.
A prototype of the modified element was tested in a vending machine for pouring water, which purified tap water in the city of Kyiv, with a salt content of 230 mg/dm3 at a temperature of 8–12 °C. The test results showed the possibility of one-stage obtaining safe physiologically complete water by reverse osmosis using a modified membrane element with the predefined selectivity of 50 %.
References
- Pro zatverdzhennia Derzhavnykh sanitarnykh norm ta pravyl "Hihienichni vymohy do vody pytnoi, pryznachenoi dlia spozhyvannia liudynoiu". Nakaz No. 400 vid 12.05.2010. Ministerstvo Okhorony Zdorovia Ukrainy. Available at: https://zakon.rada.gov.ua/laws/show/z0452-10#Text
- Directive (EU) 2020/2184 of the European Parliament and of the Council of 16 December 2020 on the quality of water intended for human consumption (recast) (Text with EEA relevance). Available at: https://eur-lex.europa.eu/eli/dir/2020/2184/oj
- National Primary Drinking Water Regulations. EPA 816-F-09-004 (2009). Available at: https://www.epa.gov/sites/default/files/2016-06/documents/npwdr_complete_table.pdf
- Guidelines for Drinking-Water Quality (2017). World Health Organization. Available at: https://www.who.int/publications/i/item/9789241549950
- Remineralizatsiya vody, ochyshchenoi systemoiu zvorotnoho osmosu. Smak vody ta zdorovyi hluzd. Available at: http://www.softwave.com.ua/remineralizatsiya-vodi-ukr/
- Vseredyni akvaboksu chystoi vody. BWT Aqua. Available at: https://bwtaqua.com.ua/inside-bwt/
- Mitchenko, T. Ye., Ponomarov, V. L., Svietlieisha, O. M., Makarova, N. V., Orestov, Ye. O., Maletskyi, Z. V. et al. (2019). Seriya vydan «Svit suchasnoi vodopidhotovky» Metody i materialy. Kyiv: VUVT WaterNet, 132.
- Filter Media. Clack. Available at: https://www.clackcorp.com/water-treatment-ion-exchange-resin-filter-media/
- Mitchenko, T. Ye., Ponomarov, V. L., Vasyliuk, S. L., Kuzminchuk, A. V., Poliakov, V. R., Stender, P. V. et al. (2021). Seriya vydan «Svit suchasnoi vodopidhotovky» Tekhnolohichni rishennia. Kyiv: VUVT WaterNet, 80.
- Lesimple, A., Ahmed, F. E., Hilal, N. (2020). Remineralization of desalinated water: Methods and environmental impact. Desalination, 496, 114692. doi: https://doi.org/10.1016/j.desal.2020.114692
- Tyvonenko, A., Mitchenko, T., Vasilyuk, S. (2022). Environmental problems caused by the use of reverse osmosis membrane elements, and ways to solve them. Water and water purification technologies. Scientific and technical news, 32 (1), 33–42. doi: https://doi.org/10.20535/2218-930012022259491
- Khaless, K., Achiou, B., Boulif, R., Benhida, R. (2021). Recycling of Spent Reverse Osmosis Membranes for Second Use in the Clarification of Wet-Process Phosphoric Acid. Minerals, 11 (6), 637. doi: https://doi.org/10.3390/min11060637
- Ouali, S., Loulergue, P., Biard, P.-F., Nasrallah, N., Szymczyk, A. (2021). Ozone compatibility with polymer nanofiltration membranes. Journal of Membrane Science, 618, 118656. doi: https://doi.org/10.1016/j.memsci.2020.118656
- Ling, R., Yu, L., Pham, T. P. T., Shao, J., Chen, J. P., Reinhard, M. (2017). The tolerance of a thin-film composite polyamide reverse osmosis membrane to hydrogen peroxide exposure. Journal of Membrane Science, 524, 529–536. doi: https://doi.org/10.1016/j.memsci.2016.11.041
- García-Pacheco, R., Landaburu-Aguirre, J., Lejarazu-Larrañaga, A., Rodríguez-Sáez, L., Molina, S., Ransome, T., García-Calvo, E. (2019). Free chlorine exposure dose (ppm•h) and its impact on RO membranes ageing and recycling potential. Desalination, 457, 133–143. doi: https://doi.org/10.1016/j.desal.2019.01.030
- Govardhan, B., Fatima, S., Madhumala, M., Sridhar, S. (2020). Modification of used commercial reverse osmosis membranes to nanofiltration modules for the production of mineral-rich packaged drinking water. Applied Water Science, 10 (11). doi: https://doi.org/10.1007/s13201-020-01312-1
- Maeda, Y. (2022). Roles of Sulfites in Reverse Osmosis (RO) Plants and Adverse Effects in RO Operation. Membranes, 12 (2), 170. doi: https://doi.org/10.3390/membranes12020170
- Antony, A., Fudianto, R., Cox, S., Leslie, G. (2010). Assessing the oxidative degradation of polyamide reverse osmosis membrane – Accelerated ageing with hypochlorite exposure. Journal of Membrane Science, 347 (1-2), 159–164. doi: https://doi.org/10.1016/j.memsci.2009.10.018
- FilmTecTM Reverse Osmosis Membranes Technical Manual. Water Solutions (2023). Available at: https://www.dupont.com/content/dam/dupont/amer/us/en/water-solutions/public/documents/en/RO-NF-FilmTec-Manual-45-D01504-en.pdf
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2023 Artem Tyvonenko, Tetiana Mitchenko, Oleksii Homaniuk, Sergey Vasilyuk, Iryna Kosogina, Rostyslav Mudryk

This work is licensed under a Creative Commons Attribution 4.0 International License.
The consolidation and conditions for the transfer of copyright (identification of authorship) is carried out in the License Agreement. In particular, the authors reserve the right to the authorship of their manuscript and transfer the first publication of this work to the journal under the terms of the Creative Commons CC BY license. At the same time, they have the right to conclude on their own additional agreements concerning the non-exclusive distribution of the work in the form in which it was published by this journal, but provided that the link to the first publication of the article in this journal is preserved.
A license agreement is a document in which the author warrants that he/she owns all copyright for the work (manuscript, article, etc.).
The authors, signing the License Agreement with TECHNOLOGY CENTER PC, have all rights to the further use of their work, provided that they link to our edition in which the work was published.
According to the terms of the License Agreement, the Publisher TECHNOLOGY CENTER PC does not take away your copyrights and receives permission from the authors to use and dissemination of the publication through the world's scientific resources (own electronic resources, scientometric databases, repositories, libraries, etc.).
In the absence of a signed License Agreement or in the absence of this agreement of identifiers allowing to identify the identity of the author, the editors have no right to work with the manuscript.
It is important to remember that there is another type of agreement between authors and publishers – when copyright is transferred from the authors to the publisher. In this case, the authors lose ownership of their work and may not use it in any way.








