Activation of nickel foam, as a current collector of a supercapacitor, by impact nickel plating: influence of treatment conditions
DOI:
https://doi.org/10.15587/1729-4061.2022.265706Keywords:
nickel foam, impact nickel, activation, current collector, supercapacitor, specific capacitance, cyclic voltammetry, charge-discharge cycling, surface developmentAbstract
Nickel foam is widely used as a current lead/current collector and as the base of nickel hydroxide electrodes for hybrid supercapacitors. An investigation of the influence of activation conditions for a commercial sample of nickel foam produced by Linyi Gelon LIB Co Ltd (China) was carried out using the method of impact nickel plating. The morphology of activated and non-activated nickel foam samples was investigated by scanning electron microscopy. Activated and non-activated nickel foam samples were investigated by methods of cyclic voltammetry and galvanostatic charge-discharge cycling in the supercapacitor mode.
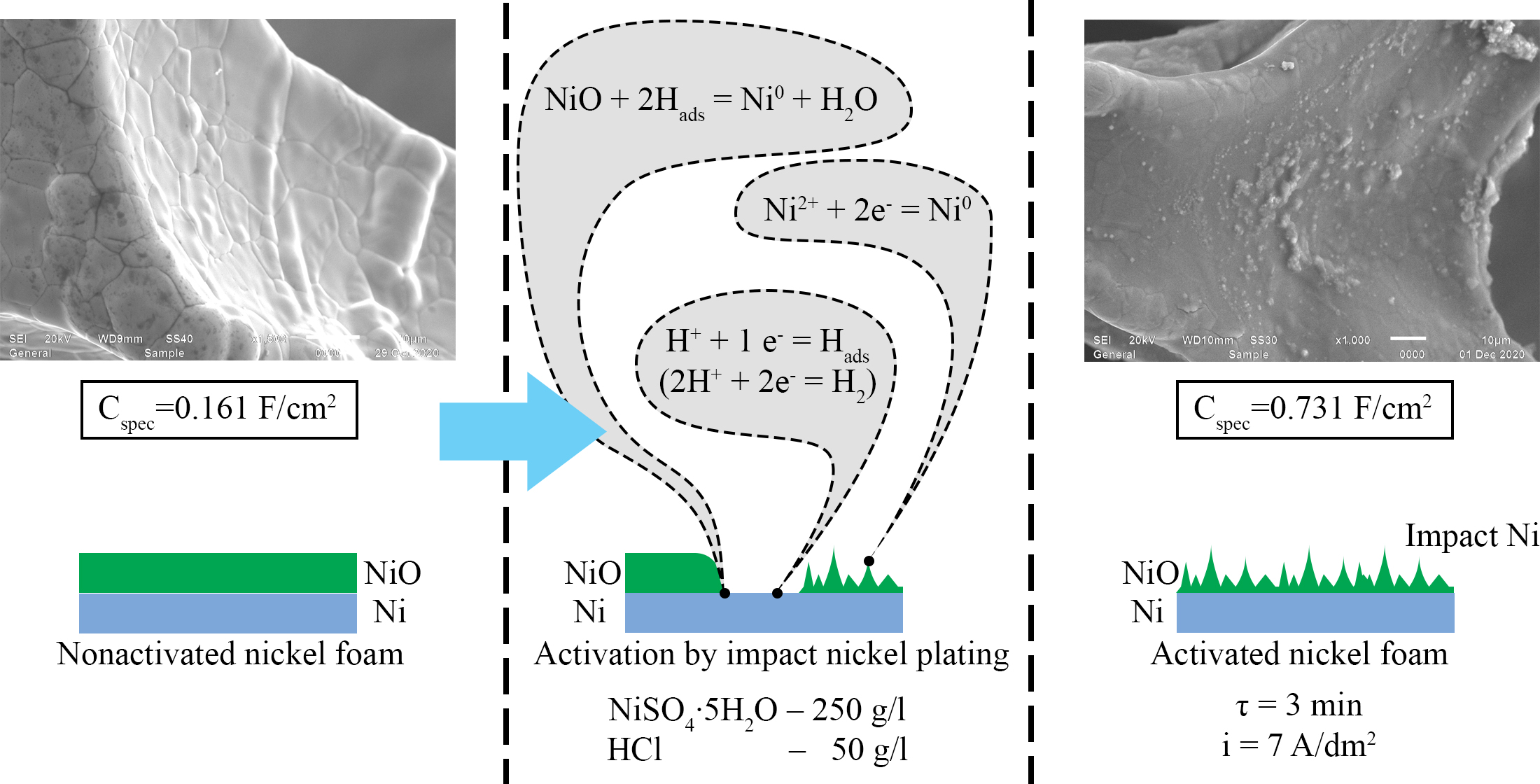
It was shown that upon activation at i=1 A/dm2 and τ=10 min, a thin layer of porous nickel with incomplete coverage was formed. Activation with impact nickel at i=7 A/dm2 and τ=3 min revealed the formation of a nickel coating with a highly developed surface, on which local cracks were found as a result of the accumulation of internal stresses. Activation with impact nickel at i=1 A/dm2 and τ=10 min led to the formation of a coating with a highly developed surface, with significant peeling of the coating.
Cyclic voltammetry showed high efficiency of impact nickel activation at i=7 A/dm2, τ=3 min, and i=20 A/dm2, τ=5 min. The specific current of the cathode peak increased 6.06–6.44 times with respect to the non-activated sample. The investigation of the activated samples' electrochemical characteristics by the galvanostatic cycling method showed that impact nickel activation at i=1 A/dm2 and τ=10 min was insufficient. It was found that at a discharge up to E=0 V, the maximum specific capacitance of 0.731 F/cm2 was obtained for samples activated by impact nickel at i=7 A/dm2 and τ=3 min. The increase in specific capacitance compared to the non-activated sample was 4.49 times. At full discharge, the highest electrochemical activity was found for nickel foam samples activated by impact nickel at i=20 A/dm2 and τ=5 min. The specific capacitance was 0.505 mA∙h/cm2, and it increased 9.02 times
References
- Medianyk, V., Cherniaiev, O. (2018). Technological aspects of technogenic disturbance liquidation in the areas of coal-gas deposits development. E3S Web of Conferences, 60, 00037. doi: https://doi.org/10.1051/e3sconf/20186000037
- Simon, P., Gogotsi, Y. (2008). Materials for electrochemical capacitors. Nature Materials, 7 (11), 845–854. doi: https://doi.org/10.1038/nmat2297
- Burke, A. (2007). R&D considerations for the performance and application of electrochemical capacitors. Electrochimica Acta, 53 (3), 1083–1091. doi: https://doi.org/10.1016/j.electacta.2007.01.011
- Lang, J.-W., Kong, L.-B., Liu, M., Luo, Y.-C., Kang, L. (2009). Asymmetric supercapacitors based on stabilized α-Ni(OH)2 and activated carbon. Journal of Solid State Electrochemistry, 14 (8), 1533–1539. doi: https://doi.org/10.1007/s10008-009-0984-1
- Lang, J.-W., Kong, L.-B., Wu, W.-J., Liu, M., Luo, Y.-C., Kang, L. (2008). A facile approach to the preparation of loose-packed Ni(OH)2 nanoflake materials for electrochemical capacitors. Journal of Solid State Electrochemistry, 13 (2), 333–340. doi: https://doi.org/10.1007/s10008-008-0560-0
- Aghazadeh, M., Ghaemi, M., Sabour, B., Dalvand, S. (2014). Electrochemical preparation of α-Ni(OH)2 ultrafine nanoparticles for high-performance supercapacitors. Journal of Solid State Electrochemistry, 18 (6), 1569–1584. doi: https://doi.org/10.1007/s10008-014-2381-7
- Zheng, C., Liu, X., Chen, Z., Wu, Z., Fang, D. (2014). Excellent supercapacitive performance of a reduced graphene oxide/Ni(OH)2 composite synthesized by a facile hydrothermal route. Journal of Central South University, 21 (7), 2596–2603. doi: https://doi.org/10.1007/s11771-014-2218-7
- Wang, B., Williams, G. R., Chang, Z., Jiang, M., Liu, J., Lei, X., Sun, X. (2014). Hierarchical NiAl Layered Double Hydroxide/Multiwalled Carbon Nanotube/Nickel Foam Electrodes with Excellent Pseudocapacitive Properties. ACS Applied Materials & Interfaces, 6 (18), 16304–16311. doi: https://doi.org/10.1021/am504530e
- Kotok, V., Кovalenko, V. (2017). Optimization of nickel hydroxide electrode of the hybrid supercapacitor. Eastern-European Journal of Enterprise Technologies, 1 (6 (85)), 4–9. doi: https://doi.org/10.15587/1729-4061.2017.90810
- Kovalenko, V. L., Kotok, V. A., Sykchin, A., Ananchenko, B. A., Chernyad’ev, A. V., Burkov, A. A. et. al. (2020). Al3+ Additive in the Nickel Hydroxide Obtained by High-Temperature Two-Step Synthesis: Activator or Poisoner for Chemical Power Source Application? Journal of The Electrochemical Society, 167 (10), 100530. doi: https://doi.org/10.1149/1945-7111/ab9a2a
- Chen, M., Xiong, X., Yi, C., Ma, J., Zeng, X. (2014). Ni(OH)2–NiO–NiF Compound Film on Nickel with Superior Pseudocapacitive Performance Prepared by Anodization and Post-hydrothermal Treatment Methods. Journal of Inorganic and Organometallic Polymers and Materials, 25 (4), 739–746. doi: https://doi.org/10.1007/s10904-014-0152-7
- Kotok, V., Kovalenko, V. (2017). The properties investigation of the faradaic supercapacitor electrode formed on foamed nickel substrate with polyvinyl alcohol using. Eastern-European Journal of Enterprise Technologies, 4 (12 (88)), 31–37. doi: https://doi.org/10.15587/1729-4061.2017.108839
- Kotok, V., Kovalenko, V., Vlasov, S. (2018). Investigation of NiAl hydroxide with silver addition as an active substance of alkaline batteries. Eastern-European Journal of Enterprise Technologies, 3 (6 (93)), 6–11. doi: https://doi.org/10.15587/1729-4061.2018.133465
- Kotok, V., Kovalenko, V. (2018). Definition of the aging process parameters for nickel hydroxide in the alkaline medium. Eastern-European Journal of Enterprise Technologies, 2 (12 (92)), 54–60. doi: https://doi.org/10.15587/1729-4061.2018.127764
- Yu, X., Hua, T., Liu, X., Yan, Z., Xu, P., Du, P. (2014). Nickel-Based Thin Film on Multiwalled Carbon Nanotubes as an Efficient Bifunctional Electrocatalyst for Water Splitting. ACS Applied Materials & Interfaces, 6 (17), 15395–15402. doi: https://doi.org/10.1021/am503938c
- Xiao, J., Zhang, X., Gao, T., Zhou, C., Xiao, D. (2017). Electrochemical formation of multilayered NiO film/Ni foam as a high-efficient anode for methanol electrolysis. Journal of Solid State Electrochemistry, 21 (8), 2301–2311. doi: https://doi.org/10.1007/s10008-017-3570-y
- Kotok, V., Kovalenko, V. (2018). A study of the effect of tungstate ions on the electrochromic properties of Ni(OH)2 films. Eastern-European Journal of Enterprise Technologies, 5 (12 (95)), 18–24. doi: https://doi.org/10.15587/1729-4061.2018.145223
- Kotok, V. A., Kovalenko, V. L. (2019). Non-Metallic Films Electroplating on the Low-Conductivity Substrates: The Conscious Selection of Conditions Using Ni(OH)2 Deposition as an Example. Journal of The Electrochemical Society, 166 (10), D395–D408. doi: https://doi.org/10.1149/2.0561910jes
- Salleh, N. A., Kheawhom, S., Mohamad, A. A. (2020). Characterizations of nickel mesh and nickel foam current collectors for supercapacitor application. Arabian Journal of Chemistry, 13 (8), 6838–6846. doi: https://doi.org/10.1016/j.arabjc.2020.06.036
- Grdeń, M., Alsabet, M., Jerkiewicz, G. (2012). Surface Science and Electrochemical Analysis of Nickel Foams. ACS Applied Materials & Interfaces, 4 (6), 3012–3021. doi: https://doi.org/10.1021/am300380m
- Solovov, V. A., Nikolenko, N. V., Kovalenko, V. L., Kotok, V. A., Burkov, A. А. et. al. (2018). Synthesis of Ni(II)-Ti(IV) Layered Double Hydroxides Using Coprecipitation At High Supersaturation Method. ARPN Journal of Engineering and Applied Sciences, 13 (24), 9652–9656. Available at: http://www.arpnjournals.org/jeas/research_papers/rp_2018/jeas_1218_7500.pdf
- Kovalenko, V., Kotok, V., Kovalenko, I. (2018). Activation of the nickel foam as a current collector for application in supercapacitors. Eastern-European Journal of Enterprise Technologies, 3 (12 (93)), 56–62. doi: https://doi.org/10.15587/1729-4061.2018.133472
- Liu, C., Huang, L., Li, Y., Sun, D. (2009). Synthesis and electrochemical performance of amorphous nickel hydroxide codoped with Fe3+ and CO32−. Ionics, 16 (3), 215–219. doi: https://doi.org/10.1007/s11581-009-0383-8
- Li, J., Luo, F., Tian, X., Lei, Y., Yuan, H., Xiao, D. (2013). A facile approach to synthesis coral-like nanoporous β-Ni(OH) 2 and its supercapacitor application. Journal of Power Sources, 243, 721–727. doi: https://doi.org/10.1016/j.jpowsour.2013.05.172
- Kovalenko, V. L., Kotok, V. A., Sykchin, A. A., Mudryi, I. A., Ananchenko, B. A., Burkov, A. A. et. al. (2016). Nickel hydroxide obtained by high-temperature two-step synthesis as an effective material for supercapacitor applications. Journal of Solid State Electrochemistry, 21 (3), 683–691. doi: https://doi.org/10.1007/s10008-016-3405-2
- Xiao-yan, G., Jian-cheng, D. (2007). Preparation and electrochemical performance of nano-scale nickel hydroxide with different shapes. Materials Letters, 61 (3), 621–625. doi: https://doi.org/10.1016/j.matlet.2006.05.026
- Kovalenko, V., Kotok, V. (2018). Synthesis of Ni(OH)2 by template homogeneous precipitation for application in the binderfree electrode of supercapacitor. Eastern-European Journal of Enterprise Technologies, 4 (12 (94)), 29–35. doi: https://doi.org/10.15587/1729-4061.2018.140899
- Tizfahm, J., Safibonab, B., Aghazadeh, M., Majdabadi, A., Sabour, B., Dalvand, S. (2014). Supercapacitive behavior of β-Ni(OH) 2 nanospheres prepared by a facile electrochemical method. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 443, 544–551. doi: https://doi.org/10.1016/j.colsurfa.2013.12.024
- Aghazadeh, M., Golikand, A. N., Ghaemi, M. (2011). Synthesis, characterization, and electrochemical properties of ultrafine β-Ni(OH)2 nanoparticles. International Journal of Hydrogen Energy, 36 (14), 8674–8679. doi: https://doi.org/10.1016/j.ijhydene.2011.03.144
- Kovalenko, V., Kotok, V. (2019). Influence of the carbonate ion on characteristics of electrochemically synthesized layered (α+β) nickel hydroxide. Eastern-European Journal of Enterprise Technologies, 1 (6 (97)), 40–46. doi: https://doi.org/10.15587/1729-4061.2019.155738
- Hall, D. S., Lockwood, D. J., Bock, C., MacDougall, B. R. (2015). Nickel hydroxides and related materials: a review of their structures, synthesis and properties. Proceedings of the Royal Society A: Mathematical, Physical and Engineering Sciences, 471 (2174), 20140792. doi: https://doi.org/10.1098/rspa.2014.0792
- Liang, K., Tang, X., Hu, W. (2012). High-performance three-dimensional nanoporous NiO film as a supercapacitor electrode. Journal of Materials Chemistry, 22 (22), 11062. doi: https://doi.org/10.1039/c2jm31526b
- Navale, S. T., Mali, V. V., Pawar, S. A., Mane, R. S., Naushad, M., Stadler, F. J., Patil, V. B. (2015). Electrochemical supercapacitor development based on electrodeposited nickel oxide film. RSC Advances, 5 (64), 51961–51965. doi: https://doi.org/10.1039/c5ra07953e
- Yuan, Y. F., Xia, X. H., Wu, J. B., Yang, J. L., Chen, Y. B., Guo, S. Y. (2011). Nickel foam-supported porous Ni(OH)2/NiOOH composite film as advanced pseudocapacitor material. Electrochimica Acta, 56 (6), 2627–2632. doi: https://doi.org/10.1016/j.electacta.2010.12.001
- Peng, H., Jing, C., Chen, J., Jiang, D., Liu, X., Dong, B. et. al. (2019). Crystal structure of nickel manganese-layered double hydroxide@cobaltosic oxides on nickel foam towards high-performance supercapacitors. CrystEngComm, 21 (3), 470–477. doi: https://doi.org/10.1039/c8ce01861h
- Nie, Y., Pan, J., Jiang, W., Pan, J., Liu, J., Sun, Y. et. al. (2020). A facile preparation of Nickel Foam-supported Ni(OH)2 nano arrays via in-situ etching method with superior bendable electrochemical performance for wearable power supply. Journal of Alloys and Compounds, 835, 155293. doi: https://doi.org/10.1016/j.jallcom.2020.155293
- Kotok, V., Kovalenko, V. (2018). A study of multilayered electrochromic platings based on nickel and cobalt hydroxides. Eastern-European Journal of Enterprise Technologies, 1 (12 (91)), 29–35. doi: https://doi.org/10.15587/1729-4061.2018.121679
- Yang, G.-W., Xu, C.-L., Li, H.-L. (2008). Electrodeposited nickel hydroxide on nickel foam with ultrahigh capacitance. Chemical Communications, 48, 6537. doi: https://doi.org/10.1039/b815647f
- Chao, Y., Xin-Bo, X., Zhi-Biao, Z., Jun-Jie, L., Tuo, H., Bin, L. et. al. (2015). Fabrication of Nickel-Based Composite Film Electrode for Supercapacitors by a New Method of Anodization/GCD. Acta Physico-Chimica Sinica, 31 (1), 99–104. doi: https://doi.org/10.3866/pku.whxb201411053
- Gu, L., Wang, Y., Lu, R., Guan, L., Peng, X., Sha, J. (2014). Anodic electrodeposition of a porous nickel oxide–hydroxide film on passivated nickel foam for supercapacitors. J. Mater. Chem. A, 2 (20), 7161–7164. doi: https://doi.org/10.1039/c4ta00205a
- Visscher, W., Barendrecht, E. (1980). The anodic oxidation of nickel in alkaline solution. Electrochimica Acta, 25 (5), 651–655. doi: https://doi.org/10.1016/0013-4686(80)87072-1
- Seghiouer, A., Chevalet, J., Barhoun, A., Lantelme, F. (1998). Electrochemical oxidation of nickel in alkaline solutions: a voltammetric study and modelling. Journal of Electroanalytical Chemistry, 442 (1-2), 113–123. doi: https://doi.org/10.1016/s0022-0728(97)00498-1
- Cai, G., Wang, X., Cui, M., Darmawan, P., Wang, J., Eh, A. L.-S., Lee, P. S. (2015). Electrochromo-supercapacitor based on direct growth of NiO nanoparticles. Nano Energy, 12, 258–267. doi: https://doi.org/10.1016/j.nanoen.2014.12.031
- Atalay, F. E., Aydogmus, E., Yigit, H., Avcu, D., Kaya, H., Atalay, S. (2014). The Formation of Free Standing NiO Nanostructures on Nickel Foam for Supercapacitors. Acta Physica Polonica A, 125 (2), 224–226. doi: https://doi.org/10.12693/aphyspola.125.224
- Yadav, A. A., Chavan, U. J. (2016). Influence of substrate temperature on electrochemical supercapacitive performance of spray deposited nickel oxide thin films. Journal of Electroanalytical Chemistry, 782, 36–42. doi: https://doi.org/10.1016/j.jelechem.2016.10.006
- Xiong, X., Zhang, J., Ma, J., Zeng, X., Qian, H., Li, Y. (2016). Fabrication of porous nickel (hydr)oxide film with rational pore size distribution on nickel foam by induction heating deposition for high-performance supercapacitors. Materials Chemistry and Physics, 181, 1–6. doi: https://doi.org/10.1016/j.matchemphys.2016.06.038
- Fares, M., Debili, M. Y. (2016). NiO Formation by Simple Air Oxidation of Nickel Coated Carbon Fibers. Journal of Advanced Microscopy Research, 11 (2), 127–129. doi: https://doi.org/10.1166/jamr.2016.1302
- Lamiel, C., Nguyen, V. H., Kumar, D. R., Shim, J.-J. (2017). Microwave-assisted binder-free synthesis of 3D Ni-Co-Mn oxide nanoflakes@Ni foam electrode for supercapacitor applications. Chemical Engineering Journal, 316, 1091–1102. doi: https://doi.org/10.1016/j.cej.2017.02.004
- Кovalenko, V., Kotok, V. (2017). Selective anodic treatment of W(WC)-based superalloy scrap. Eastern-European Journal of Enterprise Technologies, 1 (5 (85)), 53–58. doi: https://doi.org/10.15587/1729-4061.2017.91205
- Ansari, S. A., Parveen, N., Al-Othoum, M. A. S., Ansari, M. O. (2021). Effect of Washing on the Electrochemical Performance of a Three-Dimensional Current Collector for Energy Storage Applications. Nanomaterials, 11 (6), 1596. doi: https://doi.org/10.3390/nano11061596
- Bakar, N. A. A., Salleh, N. A., Hamid, N. A. A., Abdullah, C. A. C., Rahiman, W., Kheawhom, S., Mohamad, A. A. (2022). Electrochemical Characterization of Cleaning Nickel Foam Current Collector for Supercapacitor Application. Proceedings of the 7th International Corrosion Prevention Symposium for Research Scholars, 145–158. doi: https://doi.org/10.1007/978-981-19-1851-3_13
- Bakar, N. A. A., Salleh, N. A., Hamid, N. A. A., Abdullah, C. A. C., Rahiman, W., Basirun, W. J. et. al. (2022). The effect different of hydrochloric acid concentrations on the cleaning of Ni foam substrate: Structural and morphological studies. Materials Today: Proceedings, 60, 1036–1041. doi: https://doi.org/10.1016/j.matpr.2022.01.227
- Yu, D., Li, Z., Zhao, G., Zhang, H., Aslan, H., Li, J. et. al. (2019). Porous Ultrathin NiSe Nanosheet Networks on Nickel Foam for High‐Performance Hybrid Supercapacitors. ChemSusChem, 13 (1), 260–266. doi: https://doi.org/10.1002/cssc.201901766
- Kovalenko, V., Kotok, V. (2021). Comparative investigation of different types of nickel foam samples for application in supercapacitors and other electrochemical devices. Eastern-European Journal of Enterprise Technologies, 3 (12 (111)), 32–38. doi: https://doi.org/10.15587/1729-4061.2021.234251
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2022 Vadym Kovalenko, Valerii Kotok, Volodymyr Verbitskiy, Volodymyr Medianyk

This work is licensed under a Creative Commons Attribution 4.0 International License.
The consolidation and conditions for the transfer of copyright (identification of authorship) is carried out in the License Agreement. In particular, the authors reserve the right to the authorship of their manuscript and transfer the first publication of this work to the journal under the terms of the Creative Commons CC BY license. At the same time, they have the right to conclude on their own additional agreements concerning the non-exclusive distribution of the work in the form in which it was published by this journal, but provided that the link to the first publication of the article in this journal is preserved.
A license agreement is a document in which the author warrants that he/she owns all copyright for the work (manuscript, article, etc.).
The authors, signing the License Agreement with TECHNOLOGY CENTER PC, have all rights to the further use of their work, provided that they link to our edition in which the work was published.
According to the terms of the License Agreement, the Publisher TECHNOLOGY CENTER PC does not take away your copyrights and receives permission from the authors to use and dissemination of the publication through the world's scientific resources (own electronic resources, scientometric databases, repositories, libraries, etc.).
In the absence of a signed License Agreement or in the absence of this agreement of identifiers allowing to identify the identity of the author, the editors have no right to work with the manuscript.
It is important to remember that there is another type of agreement between authors and publishers – when copyright is transferred from the authors to the publisher. In this case, the authors lose ownership of their work and may not use it in any way.








