Using the hybrid composites as coating layers to inhibit the chemical corrosion in oil mineral reservoirs
DOI:
https://doi.org/10.15587/1729-4061.2022.266339Keywords:
corrosion, zinc phosphate, cellulose nitrate, coke coal, chemical and electrochemical corrosionAbstract
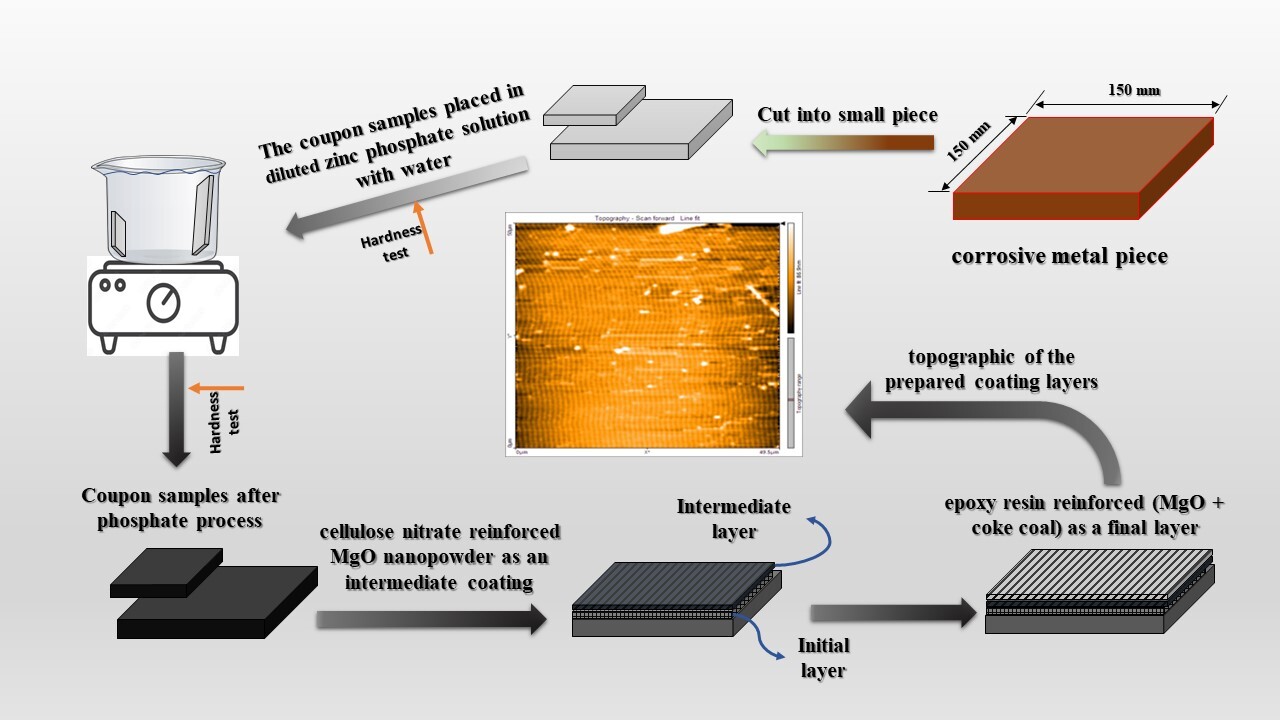
This work aims to the identify level of hybrid nanocomposite coatings of a stainless steel alloy that is used in the manufacture of mineral reservoirs for the storage of oil products in the oil products distribution company (Opdc). Corrosion is one of modern society's most serious engineering problems where losses incurred due to it each year are estimated at billions of dollars. Technological options have to be exercised to protect against corrosion and an effort to combat these losses. To overcome this severe problem, several successful efforts have been made using corrosion inhibitors. Corrosion inhibitors are compounds used in low concentrations to prevent or slow down the corrosion process. The used metal (low carbon steel St-37) was coated with zinc phosphate as an initial layer, cellulose nitrate reinforced with MgO nanopowder by weight percentage (3 wt. %) as an intermediate layer, and epoxy resin reinforced with weight reinforcement percentage (2 wt. %) of particles (MgO+coke coal (1:1)) as a final layer. In addition, a cognitive scale was prepared from (hardness, adhesion strength, chemical corrosion test as well as electrochemical corrosion test. It was found the hardness increased with coated by an initial layer and the value of adhesion strength of triple coating layers was (232 Psi). Chemical and electrochemical corrosion tests have shown the efficiency of prepared coating layers in corrosion inhibiting and metal protection. The used inhibitors in the work are inexpensive materials that allow solving the problem of rational nature management by reducing corrosion and providing the transition to the use of environmentally safe efficient technologies
References
- Gomelya, N., Trus, I., Stepova, O., Kyryliuk, O., Ivanenko, O., Homenko, A. (2020). Devising a corrosion inhibitor for steel ST37-2 in a water-oil mixture. Eastern-European Journal of Enterprise Technologies, 2 (6 (104)), 28–33. doi: https://doi.org/10.15587/1729-4061.2020.199849
- Omran, B. A., Abdel-Salam, M. O. (2020). A New Era for Microbial Corrosion Mitigation Using Nanotechnology. Springer, 201. doi: https://doi.org/10.1007/978-3-030-49532-9
- Groysman, A. (2010). Corrosion for Everybody. Springer, 368. doi: https://doi.org/10.1007/978-90-481-3477-9
- Young, D. J. (2016). High temperature oxidation and corrosion of metals. Elsevier Science. doi: https://doi.org/10.1016/C2014-0-00259-6
- Schweitzer, P. E. (2020). Metallic Materials : physical, mechanical, and corrosion properties. CRC Press, 210. Available at: https://www.routledge.com/Metallic-Materials-Physical-Mechanical-and-Corrosion-Properties/Schweitzer/p/book/9780367446888
- Lv, L.-S., Wang, J.-Y., Xiao, R.-C., Fang, M.-S., Tan, Y. (2021). Influence of steel fiber corrosion on tensile properties and cracking mechanism of ultra-high performance concrete in an electrochemical corrosion environment. Construction and Building Materials, 278, 122338. doi: https://doi.org/10.1016/j.conbuildmat.2021.122338
- Vasyliev, G., Vorobiova, V. (2019). Rape grist extract (Brassica napus) as a green corrosion inhibitor for water systems. Materials Today: Proceedings, 6, 178–186. doi: https://doi.org/10.1016/j.matpr.2018.10.092
- Khalaf, M. M., Tantawy, A. H., Soliman, K. A., Abd El-Lateef, H. M. (2020). Cationic gemini-surfactants based on waste cooking oil as new ‘green’ inhibitors for N80-steel corrosion in sulphuric acid: A combined empirical and theoretical approaches. Journal of Molecular Structure, 1203, 127442. doi: https://doi.org/10.1016/j.molstruc.2019.127442
- Liu, Y., Zhang, P. (2022). Review of Phosphorus-Based Polymers for Mineral Scale and Corrosion Control in Oilfield. Polymers, 14 (13), 2673. doi: https://doi.org/10.3390/polym14132673
- Pedeferri, P. (2018). Corrosion Science and Engineering. Springer, 720. doi: https://doi.org/10.1007/978-3-319-97625-9
- Ma, Y., Zhang, Y., Zhang, R., Guan, F., Hou, B., Duan, J. (2019). Microbiologically influenced corrosion of marine steels within the interaction between steel and biofilms: a brief view. Applied Microbiology and Biotechnology, 104 (2), 515–525. doi: https://doi.org/10.1007/s00253-019-10184-8
- O Fayomi, O. S. I., Akande, I. G., Odigie, S. (2019). Economic Impact of Corrosion in Oil Sectors and Prevention: An Overview. Journal of Physics: Conference Series, 1378 (2), 022037. doi: https://doi.org/10.1088/1742-6596/1378/2/022037
- Hasanzadeh, R., Ahmadi, J., Eghbali, M., Samadian, D., Salmanmohajer, H. (2021). Reduction of seismic resiliency of RC structures caused by chloride corrosion for typical school buildings located in hot climates. Structures, 34, 4060–4076. doi: https://doi.org/10.1016/j.istruc.2021.09.107
- Umarova, M. N., To’ychiev, A. T. (2020). Structural classification and analysis of corrosion of metals. Theoretical & Applied Science, 92 (12), 330–334. doi: https://doi.org/10.15863/tas.2020.12.92.63
- K. M. O. Goni, L., A. J. Mazumder, M. (2019). Green Corrosion Inhibitors. Corrosion Inhibitors. doi: https://doi.org/10.5772/intechopen.81376
- Talbot, D. E. J., Talbot, J. D. R. (2018). Corrosion science and technology. CRC Press, 596. Available at: https://www.routledge.com/Corrosion-Science-and-Technology/Talbot-Talbot/p/book/9781498752411
- Hao, Y., Liu, F., Han, E.-H., Anjum, S., Xu, G. (2013). The mechanism of inhibition by zinc phosphate in an epoxy coating. Corrosion Science, 69, 77–86. doi: https://doi.org/10.1016/j.corsci.2012.11.025
- Tamilselvi, M., Kamaraj, P., Arthanareeswari, M., Devikala, S. (2015). Nano zinc phosphate coatings for enhanced corrosion resistance of mild steel. Applied Surface Science, 327, 218–225. doi: https://doi.org/10.1016/j.apsusc.2014.11.081
- Bahrani, A., Naderi, R., Mahdavian, M. (2018). Chemical modification of talc with corrosion inhibitors to enhance the corrosion protective properties of epoxy-ester coating. Progress in Organic Coatings, 120, 110–122. doi: https://doi.org/10.1016/j.porgcoat.2018.03.017
- Khodair, Z. T., Khadom, A. A., Jasim, H. A. (2019). Corrosion protection of mild steel in different aqueous media via epoxy/nanomaterial coating: preparation, characterization and mathematical views. Journal of Materials Research and Technology, 8 (1), 424–435. doi: https://doi.org/10.1016/j.jmrt.2018.03.003
- Tian, Y., Huang, H., Wang, H., Xie, Y., Sheng, X., Zhong, L., Zhang, X. (2020). Accelerated formation of zinc phosphate coatings with enhanced corrosion resistance on carbon steel by introducing α-zirconium phosphate. Journal of Alloys and Compounds, 831, 154906. doi: https://doi.org/10.1016/j.jallcom.2020.154906
- Pechenkina, M. Y., Latypov, O. R., Bugai, D. E. (2021). Increasing the Corrosion Resistance of the Material of Oil and Gas Equipment in Water-Salt Solutions by Changing the Electrochemical Parameters. IOP Conference Series: Earth and Environmental Science, 720 (1), 012142. doi: https://doi.org/10.1088/1755-1315/720/1/012142
- Gupta, P., Ahamad, N., Mehta, J., Kumar, D., Quraishi, M. A., Rinawa, M. L. et al. (2021). Corrosion, optimization and surface analysis of Fe-Al2O3-CeO2 metal matrix nanocomposites. Proceedings of the Institution of Mechanical Engineers, Part C: Journal of Mechanical Engineering Science, 236 (8), 4346–4356. doi: https://doi.org/10.1177/09544062211047844
- Study the effect of the chemical heat treatments on mechanical properties steel (40 Cr) (2008). Engineering and Technology Journal, 26 (8), 324–334. Available at: https://etj.uotechnology.edu.iq/article_26705.html
- Hameed, N. A., Abbas, S. J., Jammal, M. T., Abbas, S. Q. (2022). Implementation of the MgO/epoxy nanocomposites as flame retardant. Eastern-European Journal of Enterprise Technologies, 3 (6 (117)), 53–57. doi: https://doi.org/10.15587/1729-4061.2022.260359
- Abbas, S. Q., Abd Almeer, H. A., Ahmed, W. S., Hammid, A. T. (2020). A novel algorithm for generating an edge-regular graph. Procedia Computer Science, 167, 1038–1045. doi: https://doi.org/10.1016/j.procs.2020.03.403
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2022 Noor Hameed

This work is licensed under a Creative Commons Attribution 4.0 International License.
The consolidation and conditions for the transfer of copyright (identification of authorship) is carried out in the License Agreement. In particular, the authors reserve the right to the authorship of their manuscript and transfer the first publication of this work to the journal under the terms of the Creative Commons CC BY license. At the same time, they have the right to conclude on their own additional agreements concerning the non-exclusive distribution of the work in the form in which it was published by this journal, but provided that the link to the first publication of the article in this journal is preserved.
A license agreement is a document in which the author warrants that he/she owns all copyright for the work (manuscript, article, etc.).
The authors, signing the License Agreement with TECHNOLOGY CENTER PC, have all rights to the further use of their work, provided that they link to our edition in which the work was published.
According to the terms of the License Agreement, the Publisher TECHNOLOGY CENTER PC does not take away your copyrights and receives permission from the authors to use and dissemination of the publication through the world's scientific resources (own electronic resources, scientometric databases, repositories, libraries, etc.).
In the absence of a signed License Agreement or in the absence of this agreement of identifiers allowing to identify the identity of the author, the editors have no right to work with the manuscript.
It is important to remember that there is another type of agreement between authors and publishers – when copyright is transferred from the authors to the publisher. In this case, the authors lose ownership of their work and may not use it in any way.








