Effect of syzygium cumini leaf extract as a green corrosion inhibitor on API 5l carbon steel in 1M HCL
DOI:
https://doi.org/10.15587/1729-4061.2022.267232Keywords:
green corrosion inhibitor, Syzygium cumini leaf extract, physisorption, polyphenol and flavonoids based inhibitor moleculesAbstract
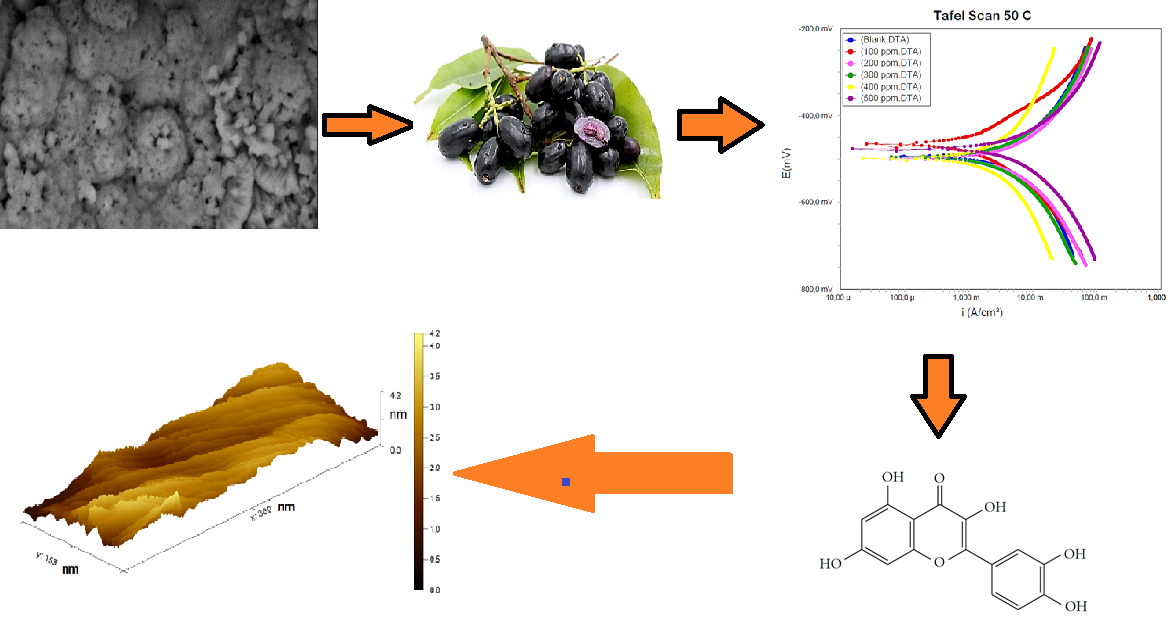
Corrosion in API 5L steel under 1M HCl is a common issue; hence, creating a more effective and naturally-based inhibitor is critical. In this research, Syzygium Cumini leaf extract (SCLE) was used as a new green corrosion inhibitor under acidic conditions. The inhibition properties of the novel cumini extract were thoroughly characterized using potentiodynamic polarization (PDP), electrochemical impedance spectroscopy (EIS), Fourier-transform infrared spectroscopy (FTIR), and atomic force microscope (AFM). The results show that the cumini inhibitor has excellent corrosion inhibition with 93 % inhibition efficiency. The adsorption behavior of the inhibitor follows the Langmuir Adsorption Isotherm due to the nearness of R2 to unity. The potentiodynamic and electrochemical measurements demonstrate the mixed type of corrosion inhibitor. Thermodynamic calculation of ΔGads is – 18.41 kJ mol-1 showing the physical adsorption process between the inhibitor and metals. Further inspection of ΔHads at ‒58.93 kJ mol-1 considers releasing energy during adsorption. The FTIR results agree with the increased growth of passive layers due to the adsorption of polyphenol and flavonoids on metals. Remarkably, the adsorption peak at 3266.59 cm-1 corresponds to the adsorption of –OH. The peak at 1612.56 and 1698.4 cm-1 is attributed to C=C and C=O functional groups. The above functional groups serve as adsorption centers to reduce the corrosion effect. The surface treatment of AFM indicated a good relationship with the functional group characterization and confirmed the significant corrosion rate reduction. This work can be used as a benchmark to develop a natural plant as a corrosion inhibitor.
Supporting Agency
- The author gratefully thanks the Ministry of Research, Technology and Higher Education Indonesia for International Indexed Publication Grants (PUTI) Q2 Financial Year of 2022-2023 Batch 3- Matching Fund (MF) Financial Year of 2022—2023, with contract number: NKB-1488/UN2.RST/HKP.05.00/2022.
References
- Neriyana, P. S., Alva, V. D. P. (2020). A Green Approach: Evaluation of Combretum indicum (CI) Leaf Extract as an Eco-friendly Corrosion Inhibitor for Mild Steel in 1M HCl. Chemistry Africa, 3 (4), 1087–1098. doi: https://doi.org/10.1007/s42250-020-00190-z
- Salmasifar, A., Edraki, M., Alibakhshi, E., Ramezanzadeh, B., Bahlakeh, G. (2021). Combined electrochemical/surface investigations and computer modeling of the aquatic Artichoke extract molecules corrosion inhibition properties on the mild steel surface immersed in the acidic medium. Journal of Molecular Liquids, 327, 114856. doi: https://doi.org/10.1016/j.molliq.2020.114856
- Prifiharni, S., Mashanafie, G., Priyotomo, G., Royani, A., Ridhova, A., Elya, B., Soedarsono, J. W. (2022). Extract sarampa wood (Xylocarpus Moluccensis) as an eco-friendly corrosion inhibitor for mild steel in HCl 1M. Journal of the Indian Chemical Society, 99 (7), 100520. doi: https://doi.org/10.1016/j.jics.2022.100520
- Abba, B. N., Idouhli, R., Ilagouma, A. T., Abouelfida, A., Khadiri, M., Romane, A. (2021). Use of Endostemon tereticaulis (Pear.) M.Ashby and Hyptis spicigera Lam. Plant Extracts as Corrosion Green Inhibitors for Mild Steel in 1M HCl: Electrochemical and Surface Morphological Studies. Protection of Metals and Physical Chemistry of Surfaces, 57 (3), 619–633. doi: https://doi.org/10.1134/s2070205121030035
- Rajan, J. P., Shrivastava, R., Mishra, R. K. (2017). Corrosion Inhibition effect of Clerodendron Colebrookianum Walp Leaves (Phuinam) Extract on the Acid Corrosion of Mild Steel. Protection of Metals and Physical Chemistry of Surfaces, 53 (6), 1161–1172. doi: https://doi.org/10.1134/s2070205118010264
- Sun, X., Qiang, Y., Hou, B., Zhu, H., Tian, H. (2022). Cabbage extract as an eco-friendly corrosion inhibitor for X70 steel in hydrochloric acid medium. Journal of Molecular Liquids, 362, 119733. doi: https://doi.org/10.1016/j.molliq.2022.119733
- Fouda, A. S., El-Awady, G. Y., El Behairy, W. T. (2017). Prosopis juliflora Plant Extract as Potential Corrosion Inhibitor for Low-Carbon Steel in 1 M HCl Solution. Journal of Bio- and Tribo-Corrosion, 4 (1). doi: https://doi.org/10.1007/s40735-017-0124-x
- Perumal, S., Muthumanickam, S., Elangovan, A., Karthik, R., kannan, R. S., Mothilal, K. K. (2017). Bauhinia tomentosa Leaves Extract as Green Corrosion Inhibitor for Mild Steel in 1M HCl Medium. Journal of Bio- and Tribo-Corrosion, 3 (2). doi: https://doi.org/10.1007/s40735-017-0072-5
- Khadom, A. A., Abd, A. N., Ahmed, N. A., Kadhim, M. M., Fadhil, A. A. (2022). Combined influence of iodide ions and Xanthium Strumarium leaves extract as eco-friendly corrosion inhibitor for low-carbon steel in hydrochloric acid. Current Research in Green and Sustainable Chemistry, 5, 100278. doi: https://doi.org/10.1016/j.crgsc.2022.100278
- Zakaria, F. A., Hamidon, T. S., Hussin, M. H. (2022). Applicability of winged bean extracts as organic corrosion inhibitors for reinforced steel in 0.5 M HCl electrolyte. Journal of the Indian Chemical Society, 99 (2), 100329. doi: https://doi.org/10.1016/j.jics.2021.100329
- Raghavendra, N. (2019). Latest Exploration on Natural Corrosion Inhibitors for Industrial Important Metals in Hostile Fluid Environments: A Comprehensive Overview. Journal of Bio- and Tribo-Corrosion, 5 (3). doi: https://doi.org/10.1007/s40735-019-0240-x
- Dehghani, A., Ghahremani, P., Mostafatabar, A. H., Ramezanzadeh, B. (2022). Plant extracts: Probable alternatives for traditional inhibitors for controlling alloys corrosion against acidic media – A review. Biomass Conversion and Biorefinery. doi: https://doi.org/10.1007/s13399-022-02893-4
- Panchal, J., Shah, D., Patel, R., Shah, S., Prajapati, M., Shah, M. (2021). Comprehensive Review and Critical Data Analysis on Corrosion and Emphasizing on Green Eco-friendly Corrosion Inhibitors for Oil and Gas Industries. Journal of Bio- and Tribo-Corrosion, 7 (3). doi: https://doi.org/10.1007/s40735-021-00540-5
- Chaubey, N., Savita, Qurashi, A., Chauhan, D. S., Quraishi, M. A. (2021). Frontiers and advances in green and sustainable inhibitors for corrosion applications: A critical review. Journal of Molecular Liquids, 321, 114385. doi: https://doi.org/10.1016/j.molliq.2020.114385
- Chauhan, D. S., Quraishi, M. A., Qurashi, A. (2021). Recent trends in environmentally sustainable Sweet corrosion inhibitors. Journal of Molecular Liquids, 326, 115117. doi: https://doi.org/10.1016/j.molliq.2020.115117
- Jmiai, A., El Ibrahimi, B., Tara, A., El Issami, S., Jbara, O., Bazzi, L. (2018). Alginate biopolymer as green corrosion inhibitor for copper in 1 M hydrochloric acid: Experimental and theoretical approaches. Journal of Molecular Structure, 1157, 408–417. doi: https://doi.org/10.1016/j.molstruc.2017.12.060
- Kamali Ardakani, E., Kowsari, E., Ehsani, A. (2020). Imidazolium-derived polymeric ionic liquid as a green inhibitor for corrosion inhibition of mild steel in 1.0 M HCl: Experimental and computational study. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 586, 124195. doi: https://doi.org/10.1016/j.colsurfa.2019.124195
- Abaka, A. K., Ishaku, G. A., Haruna, A., Ardo, B. P. (2020). Phytochemicals Screening and Antifungal Activity of Balanites aegyptiaca Seed and Callus Extract against Candida albicans. Asian Plant Research Journal, 4 (4), 9–16. doi: https://doi.org/10.9734/aprj/2020/v4i430091
- Fang, Y., Suganthan, B., Ramasamy, R. P. (2019). Electrochemical characterization of aromatic corrosion inhibitors from plant extracts. Journal of Electroanalytical Chemistry, 840, 74–83. doi: https://doi.org/10.1016/j.jelechem.2019.03.052
- Fouda, A. S., Mohamed, O. A., Elabbasy, H. M. (2021). Ferula hermonis Plant Extract as Safe Corrosion Inhibitor for Zinc in Hydrochloric Acid Solution. Journal of Bio- and Tribo-Corrosion, 7 (4). doi: https://doi.org/10.1007/s40735-021-00570-z
- Lohitkarn, J., Hemwech, P., Chantiwas, R., Jariyaboon, M. (2021). The Role of Cassava Leaf Extract as Green Inhibitor for Controlling Corrosion and Scale Problems in Cooling Water Systems. Journal of Failure Analysis and Prevention. doi: https://doi.org/10.1007/s11668-021-01121-x
- Rustandi, A., Soedarsono, J. W., Suharno, B. (2011). The Use of Mixture of Piper Betle and Green Tea as a Green Corrosion Inhibitor for API X-52 Steel in Aerated 3.5 % NaCl Solution at Various Rotation Rates. Advanced Materials Research, 383–390, 5418–5425. doi: https://doi.org/10.4028/www.scientific.net/amr.383-390.5418
- Huong Pham, T., Lee, W.-H., Kim, J.-G. (2022). Chrysanthemum coronarium leaves extract as an eco-friendly corrosion inhibitor for aluminum anode in aluminum-air battery. Journal of Molecular Liquids, 347, 118269. doi: https://doi.org/10.1016/j.molliq.2021.118269
- Umoren, S. A., Solomon, M. M., Obot, I. B., Suleiman, R. K. (2021). Date palm leaves extract as a green and sustainable corrosion inhibitor for low carbon steel in 15 wt.% HCl solution: the role of extraction solvent on inhibition effect. Environmental Science and Pollution Research, 28 (30), 40879–40894. doi: https://doi.org/10.1007/s11356-021-13567-5
- Pramana, R. I., Kusumastuti, R., Soedarsono, J. W., Rustandi, A. (2013). Corrosion Inhibition of Low Carbon Steel by Pluchea Indica Less. in 3.5% NaCL Solution. Advanced Materials Research, 785–786, 20–24. doi: https://doi.org/10.4028/www.scientific.net/amr.785-786.20
- Wang, Q., Wu, X., Zheng, H., Xiao, X., Liu, L., Zhang, Q. et al. (2022). Insight into anti-corrosion behavior of Centipeda minima leaves extract as high-efficiency and eco-friendly inhibitor. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 640, 128458. doi: https://doi.org/10.1016/j.colsurfa.2022.128458
- Okeniyi, J. O., Ogbiye, A. S., Ogunlana, O. O., Okeniyi, E. T., Ogunlana, O. E. (2015). Investigating Solanum Aethiopicum Leaf-Extract and Sodium-Dichromate Effects on Steel-Rebar Corrosion in Saline/Marine Simulating-Environment: Implications on Sustainable Alternative for Environmentally-Hazardous Inhibitor. Engineering Solutions for Sustainability, 167–175. doi: https://doi.org/10.1007/978-3-319-48138-8_16
- Kaban, A. P. S., Ridhova, A., Priyotomo, G., Elya, B., Maksum, A., Sadeli, Y. et al. (2021). Development of white tea extract as green corrosion inhibitor in mild steel under 1 M hydrochloric acid solution. Eastern-European Journal of Enterprise Technologies, 2 (6 (110)), 6–20. doi: https://doi.org/10.15587/1729-4061.2021.224435
- Popoola, L. T. (2019). Organic green corrosion inhibitors (OGCIs): a critical review. Corrosion Reviews, 37 (2), 71–102. doi: https://doi.org/10.1515/corrrev-2018-0058
- Aslam, R., Mobin, M., Aslam, J., Lgaz, H., Chung, I.-M. (2019). Inhibitory effect of sodium carboxymethylcellulose and synergistic biodegradable gemini surfactants as effective inhibitors for MS corrosion in 1 M HCl. Journal of Materials Research and Technology, 8 (5), 4521–4533. doi: https://doi.org/10.1016/j.jmrt.2019.07.065
- Izadi, M., Shahrabi, T., Ramezanzadeh, B. (2017). Electrochemical investigations of the corrosion resistance of a hybrid sol–gel film containing green corrosion inhibitor-encapsulated nanocontainers. Journal of the Taiwan Institute of Chemical Engineers, 81, 356–372. doi: https://doi.org/10.1016/j.jtice.2017.10.039
- Farahati, R., Mousavi-Khoshdel, S. M., Ghaffarinejad, A., Behzadi, H. (2020). Experimental and computational study of penicillamine drug and cysteine as water-soluble green corrosion inhibitors of mild steel. Progress in Organic Coatings, 142, 105567. doi: https://doi.org/10.1016/j.porgcoat.2020.105567
- Paul Setiawan Kaban, A., Mayangsari, W., Syaiful Anwar, M., Maksum, A., Riastuti, R. et al. (2022). Experimental and modelling waste rice husk ash as a novel green corrosion inhibitor under acidic environment. Materials Today: Proceedings, 62, 4225–4234. doi: https://doi.org/10.1016/j.matpr.2022.04.738
- Hajipour, F., Asad, S., Amoozegar, M. A., Javidparvar, A. A., Tang, J., Zhong, H., Khajeh, K. (2021). Developing a Fluorescent Hybrid Nanobiosensor Based on Quantum Dots and Azoreductase Enzyme forMethyl Red Monitoring. Iranian Biomedical Journal, 25 (1), 8–20. doi: https://doi.org/10.29252/ibj.25.1.8
- Javidparvar, A. A., Naderi, R., Ramezanzadeh, B., Bahlakeh, G. (2019). Graphene oxide as a pH-sensitive carrier for targeted delivery of eco-friendly corrosion inhibitors in chloride solution: Experimental and theroretical investigations. Journal of Industrial and Engineering Chemistry, 72, 196–213. doi: https://doi.org/10.1016/j.jiec.2018.12.019
- Ravari, F. B., Dadgareenezhad, A. (2013). Synergistic influence of propargyl alcohol and zinc sulfate on inhibition of corrosion of aluminum in 0.5M H2So4. Journal of the Chilean Chemical Society, 58 (3), 1853–1857. doi: https://doi.org/10.4067/s0717-97072013000300013
- Fragoza-Mar, L., Olivares-Xometl, O., Domínguez-Aguilar, M. A., Flores, E. A., Arellanes-Lozada, P., Jiménez-Cruz, F. (2012). Corrosion inhibitor activity of 1,3-diketone malonates for mild steel in aqueous hydrochloric acid solution. Corrosion Science, 61, 171–184. doi: https://doi.org/10.1016/j.corsci.2012.04.031
- Hamani, H., Douadi, T., Al-Noaimi, M., Issaadi, S., Daoud, D., Chafaa, S. (2014). Electrochemical and quantum chemical studies of some azomethine compounds as corrosion inhibitors for mild steel in 1M hydrochloric acid. Corrosion Science, 88, 234–245. doi: https://doi.org/10.1016/j.corsci.2014.07.044
- Lai, C., Xie, B., Zou, L., Zheng, X., Ma, X., Zhu, S. (2017). Adsorption and corrosion inhibition of mild steel in hydrochloric acid solution by S-allyl-O,O′-dialkyldithiophosphates. Results in Physics, 7, 3434–3443. doi: https://doi.org/10.1016/j.rinp.2017.09.012
- Godwin-Nwakwasi, E. U., Elachi, E. E., Ezeokonkwo, M. A., Onwuchuruba, L. E. (2017). A Study of the Corrosion Inhibition of Mild Steel in 0.5M Tetraoxosulphate (VI) acid by Alstonia boonei Leaves Extract as an Inhibitor at Different Temperatures. International Journal of Advanced Engineering, Management and Science, 3 (12), 1150–1157. doi: https://doi.org/10.22161/ijaems.3.12.9
- Hart, E. (Ed.) (2016). Corrosion inhibitors: Principles, mechanisms and applications. Nova Science Publishers, Inc.
- Aourabi, S., Driouch, M., Sfaira, M., Mahjoubi, F., Hammouti, B., Verma, C. et al. (2021). Phenolic fraction of Ammi visnaga extract as environmentally friendly antioxidant and corrosion inhibitor for mild steel in acidic medium. Journal of Molecular Liquids, 323, 114950. doi: https://doi.org/10.1016/j.molliq.2020.114950
- Xhanari, K., Finšgar, M., Knez Hrnčič, M., Maver, U., Knez, Ž., Seiti, B. (2017). Green corrosion inhibitors for aluminium and its alloys: a review. RSC Advances, 7 (44), 27299–27330. doi: https://doi.org/10.1039/c7ra03944a
- Li, X., Deng, S., Fu, H., Xie, X. (2014). Synergistic inhibition effects of bamboo leaf extract/major components and iodide ion on the corrosion of steel in H3PO4 solution. Corrosion Science, 78, 29–42. doi: https://doi.org/10.1016/j.corsci.2013.08.025
- Xu, Y. et al. (2018). Halogen-substituted pyrazolo-pyrimidine derivatives as corrosion inhibitors for copper in sulfuric acid solution. International Journal of Corrosion and Scale Inhibition, 7 (2). doi: https://doi.org/10.17675/2305-6894-2018-7-2-9
- Kim, S., Jang, Y., Sung, S., Kim, Y. (2007). Inhibitory Activity of Phenolic Glycosides from the Fruits of Idesia polycarpa on Lipopolysaccharide-Induced Nitric Oxide Production in BV2 Microglia. Planta Medica, 73 (2), 167–169. doi: https://doi.org/10.1055/s-2006-951771
- Shahzad, K., Sliem, M. H., Shakoor, R. A., Radwan, A. B., Kahraman, R., Umer, M. A. et al. (2020). Electrochemical and thermodynamic study on the corrosion performance of API X120 steel in 3.5% NaCl solution. Scientific Reports, 10 (1). doi: https://doi.org/10.1038/s41598-020-61139-3
- Tan, B., Zhang, S., Qiang, Y., Li, W., Li, H., Feng, L. et al. (2020). Experimental and theoretical studies on the inhibition properties of three diphenyl disulfide derivatives on copper corrosion in acid medium. Journal of Molecular Liquids, 298, 111975. doi: https://doi.org/10.1016/j.molliq.2019.111975
- Sedik, A., Lerari, D., Salci, A., Athmani, S., Bachari, K., Gecibesler, İ. H., Solmaz, R. (2020). Dardagan Fruit extract as eco-friendly corrosion inhibitor for mild steel in 1 M HCl: Electrochemical and surface morphological studies. Journal of the Taiwan Institute of Chemical Engineers, 107, 189–200. doi: https://doi.org/10.1016/j.jtice.2019.12.006
- Miralrio, A., Espinoza Vázquez, A. (2020). Plant Extracts as Green Corrosion Inhibitors for Different Metal Surfaces and Corrosive Media: A Review. Processes, 8 (8), 942. doi: https://doi.org/10.3390/pr8080942
- Ricky, E. X., Mpelwa, M., Xu, X. (2021). The study of m-pentadecylphenol on the inhibition of mild steel corrosion in 1 M HCl solution. Journal of Industrial and Engineering Chemistry, 101, 359–371. doi: https://doi.org/10.1016/j.jiec.2021.05.047
- Al-Ghouti, M. A., Da’ana, D. A. (2020). Guidelines for the use and interpretation of adsorption isotherm models: A review. Journal of Hazardous Materials, 393, 122383. doi: https://doi.org/10.1016/j.jhazmat.2020.122383
- Vashishth, P., Bairagi, H., Narang, R., Shukla, S. K., Mangla, B. (2022). Thermodynamic and electrochemical investigation of inhibition efficiency of green corrosion inhibitor and its comparison with synthetic dyes on MS in acidic medium. Journal of Molecular Liquids, 365, 120042. doi: https://doi.org/10.1016/j.molliq.2022.120042
- Noorbakhsh Nezhad, A. H., Davoodi, A., Mohammadi Zahrani, E., Arefinia, R. (2020). The effects of an inorganic corrosion inhibitor on the electrochemical behavior of superhydrophobic micro-nano structured Ni films in 3.5% NaCl solution. Surface and Coatings Technology, 395, 125946. doi: https://doi.org/10.1016/j.surfcoat.2020.125946
- Chauhan, D. S., Quraishi, M. A., Srivastava, V., Haque, J., ibrahimi, B. E. (2021). Virgin and chemically functionalized amino acids as green corrosion inhibitors: Influence of molecular structure through experimental and in silico studies. Journal of Molecular Structure, 1226, 129259. doi: https://doi.org/10.1016/j.molstruc.2020.129259
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2022 Rini Riastuti, Giannisa Mashanafie, Vika Rizkia, Ahmad Maksum, Siska Prifiharni, Agus Kaban, Gadang Priyotomo, Johny Soedarsono

This work is licensed under a Creative Commons Attribution 4.0 International License.
The consolidation and conditions for the transfer of copyright (identification of authorship) is carried out in the License Agreement. In particular, the authors reserve the right to the authorship of their manuscript and transfer the first publication of this work to the journal under the terms of the Creative Commons CC BY license. At the same time, they have the right to conclude on their own additional agreements concerning the non-exclusive distribution of the work in the form in which it was published by this journal, but provided that the link to the first publication of the article in this journal is preserved.
A license agreement is a document in which the author warrants that he/she owns all copyright for the work (manuscript, article, etc.).
The authors, signing the License Agreement with TECHNOLOGY CENTER PC, have all rights to the further use of their work, provided that they link to our edition in which the work was published.
According to the terms of the License Agreement, the Publisher TECHNOLOGY CENTER PC does not take away your copyrights and receives permission from the authors to use and dissemination of the publication through the world's scientific resources (own electronic resources, scientometric databases, repositories, libraries, etc.).
In the absence of a signed License Agreement or in the absence of this agreement of identifiers allowing to identify the identity of the author, the editors have no right to work with the manuscript.
It is important to remember that there is another type of agreement between authors and publishers – when copyright is transferred from the authors to the publisher. In this case, the authors lose ownership of their work and may not use it in any way.








