Development of granular composites based on laponite and Zr/Fe-alginate for effective removal of uranium (VI) from sulfate solutions
DOI:
https://doi.org/10.15587/1729-4061.2023.292524Keywords:
granular composites, zirconium-iron alginates, uranium (VI) removal, sulfate solutions, laponiteAbstract
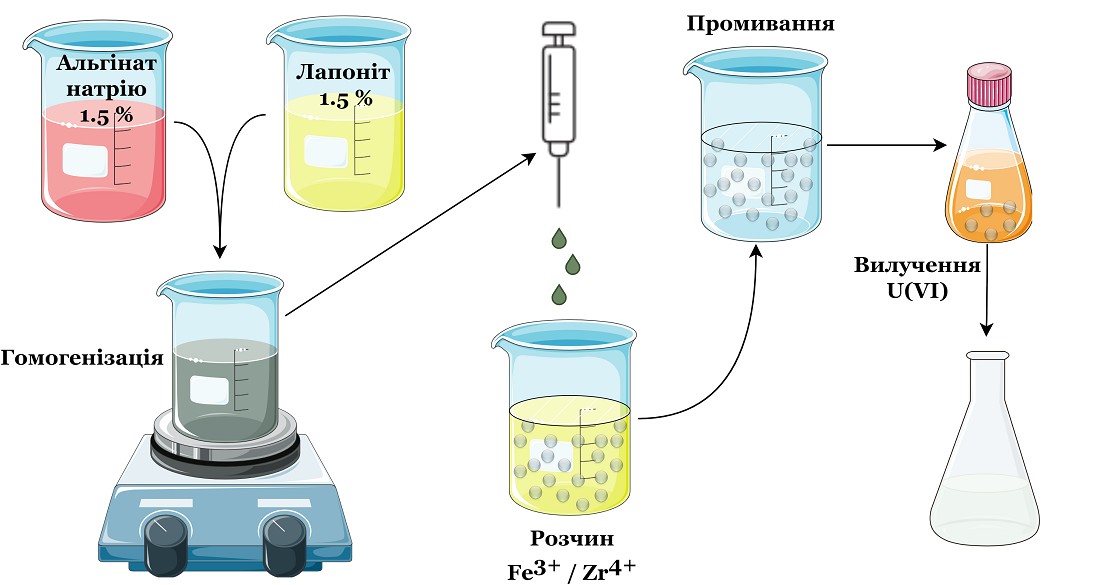
The object of research is granular composites based on zirconium-iron alginates and laponite. The task of research is to determine the influence of the Zr:Fe ratio on the structure of granular composites and efficiency of uranium (VI) removal from aqueous solutions. The influence of the zirconium and iron ratio on the parameters of the material's pore structure has been established, particularly on the change in the content of micropores within the matrix. The specific surface area of the materials ranges from 86 to 112 m²/g. The sorption properties of the composites regarding uranium (VI) have been investigated. The impact of the charge of surface groups and the form of uranium (VI) presence in sulfate solutions on their sorption characteristics has been demonstrated. The maximum adsorption capacity reaches 265.1 µmol/g at pH 6. It is shown that an elevated electrolyte content positively affects the efficiency of uranium (VI) removal in neutral and alkaline medium. It has been established that structural changes in the materials occur due to the intensive interaction of iron ions and alginate molecules, resulting in the formation of a dense gel-like structure. The mechanism of uranium (VI) removal is associated with the formation of surface complexes in the presence of electrolytes. The synthesized granulated composites exhibit improved removal efficiency of uranium (VI) under conditions of high mineralization of solutions, making them attractive for potential use as sorbents. The obtained results can be utilized in the development of effective methods for purifying water environments from uranium (VI) in high mineralization conditions, which is a relevant issue in the field of nuclear energy and the removal of radioactive substances from water systems
References
- Deng, D., Zhang, L., Dong, M., Samuel, R. E., Ofori‐Boadu, A., Lamssali, M. (2020). Radioactive waste: A review. Water Environment Research, 92 (10), 1818–1825. doi: https://doi.org/10.1002/wer.1442
- Bachmaf, S., Planer-Friedrich, B., Merkel, B. J. (2008). Effect of sulfate, carbonate, and phosphate on the uranium(VI) sorption behavior onto bentonite. Radiochimica Acta, 96 (6), 359–366. doi: https://doi.org/10.1524/ract.2008.1496
- Pylypenko, I., Spasоnova, L., Kovalchuk, I., Veremeienko, V. (2014). Sorption of cobalt, chromium and uranium ions on Fe/Ti-pillared montmorillonite. Eastern-European Journal of Enterprise Technologies, 4 (6 (70)), 57–61. doi: https://doi.org/10.15587/1729-4061.2014.26246
- Liu, W., Wang, Q., Wang, H., Xin, Q., Hou, W., Hu, E., Lei, Z. (2022). Adsorption of uranium by chitosan/Chlorella pyrenoidosa composite adsorbent bearing phosphate ligand. Chemosphere, 287, 132193. doi: https://doi.org/10.1016/j.chemosphere.2021.132193
- Algothmi, W. M., Bandaru, N. M., Yu, Y., Shapter, J. G., Ellis, A. V. (2013). Alginate–graphene oxide hybrid gel beads: An efficient copper adsorbent material. Journal of Colloid and Interface Science, 397, 32–38. doi: https://doi.org/10.1016/j.jcis.2013.01.051
- Gao, X., Li, M., Zhao, Y., Zhang, Y. (2019). Mechanistic study of selective adsorption of Hg2+ ion by porous alginate beads. Chemical Engineering Journal, 378, 122096. doi: https://doi.org/10.1016/j.cej.2019.122096
- Shawky, H. A. (2010). Improvement of water quality using alginate/montmorillonite composite beads. Journal of Applied Polymer Science, 119 (4), 2371–2378. doi: https://doi.org/10.1002/app.32694
- da Silva Fernandes, R., de Moura, M. R., Glenn, G. M., Aouada, F. A. (2018). Thermal, microstructural, and spectroscopic analysis of Ca2+ alginate/clay nanocomposite hydrogel beads. Journal of Molecular Liquids, 265, 327–336. doi: https://doi.org/10.1016/j.molliq.2018.06.005
- Kumar, S., Dumpala, R. M. R., Chandane, A., Bahadur, J. (2022). Elucidation of the sorbent role in sorption thermodynamics of uranium(VI) on goethite. Environmental Science: Processes & Impacts, 24 (4), 567–575. doi: https://doi.org/10.1039/d1em00380a
- Liu, H., Wang, R., Jiang, H., Gong, H., Wu, X. (2015). Study on adsorption characteristics of uranyl ions from aqueous solutions using zirconium hydroxide. Journal of Radioanalytical and Nuclear Chemistry, 308 (1), 213–220. doi: https://doi.org/10.1007/s10967-015-4315-y
- Gao, X., Guo, C., Hao, J., Zhao, Z., Long, H., Li, M. (2020). Adsorption of heavy metal ions by sodium alginate based adsorbent-a review and new perspectives. International Journal of Biological Macromolecules, 164, 4423–4434. doi: https://doi.org/10.1016/j.ijbiomac.2020.09.046
- Wang, D., Zhang, J., Li, J. (2023). Phosphate-functionalized magnetic calcium alginate for the engineering remediation of uranium-contaminated water and soil. Chemical Engineering Journal, 475, 145910. doi: https://doi.org/10.1016/j.cej.2023.145910
- Xie, S. B., Luo, J., Liu, Q., Ling, H., Duan, Y., Wang, J. (2015). Adsorption characteristics and mechanism of hydroxyethyl cellulose/sodium alginate blend films for uranium (VI). Acta Materiae Compositae Sinica, 32 (1), 268–275. doi: https://doi.org/10.13801/j.cnki.fhclxb.20140519.002
- Yi, X., Sun, F., Han, Z., Han, F., He, J., Ou, M. et al. (2018). Graphene oxide encapsulated polyvinyl alcohol/sodium alginate hydrogel microspheres for Cu (II) and U (VI) removal. Ecotoxicology and Environmental Safety, 158, 309–318. doi: https://doi.org/10.1016/j.ecoenv.2018.04.039
- Wen, S., Wang, H., Xin, Q., Hu, E., Lei, Z., Hu, F., Wang, Q. (2023). Selective adsorption of uranium (VI) from wastewater using a UiO-66/calcium alginate/hydrothermal carbon composite material. Carbohydrate Polymers, 315, 120970. doi: https://doi.org/10.1016/j.carbpol.2023.120970
- Tripathi, A., Melo, J. S., D’Souza, S. F. (2013). Uranium (VI) recovery from aqueous medium using novel floating macroporous alginate-agarose-magnetite cryobeads. Journal of Hazardous Materials, 246-247, 87–95. doi: https://doi.org/10.1016/j.jhazmat.2012.12.002
- Yu, J., Wang, J., Jiang, Y. (2017). Removal of Uranium from Aqueous Solution by Alginate Beads. Nuclear Engineering and Technology, 49 (3), 534–540. doi: https://doi.org/10.1016/j.net.2016.09.004
- Qing, Z., Wang, L., Liu, X., Song, Z., Qian, F., Song, Y. (2022). Simply synthesized sodium alginate/zirconium hydrogel as adsorbent for phosphate adsorption from aqueous solution: Performance and mechanisms. Chemosphere, 291, 133103. doi: https://doi.org/10.1016/j.chemosphere.2021.133103
- Yu, L., Ma, Y., Ong, C. N., Xie, J., Liu, Y. (2015). Rapid adsorption removal of arsenate by hydrous cerium oxide–graphene composite. RSC Advances, 5 (80), 64983–64990. doi: https://doi.org/10.1039/c5ra08922k
- Rouquerol, F., Rouquerol, J. et al. (2014). Adsorption by Powders and Porous Solids. Elsevier. doi: https://doi.org/10.1016/c2010-0-66232-8
- Doroshenko, D., Pylypenko, I., Kovalchuk, I., Kornilovych, B., Spasonova, L. (2018). Investigation of the structure and sorption peculiarities of cobalt and uranium ions by nanocomposites based on montmorillonite and tetraethoxysilane. Eastern-European Journal of Enterprise Technologies, 5 (6 (95)), 6–11. doi: https://doi.org/10.15587/1729-4061.2018.144553
- Swain, S. K., Patnaik, T., Patnaik, P. C., Jha, U., Dey, R. K. (2013). Development of new alginate entrapped Fe(III)–Zr(IV) binary mixed oxide for removal of fluoride from water bodies. Chemical Engineering Journal, 215-216, 763–771. doi: https://doi.org/10.1016/j.cej.2012.10.098
- Yu, S., Ma, J., Shi, Y., Du, Z., Zhao, Y., Tuo, X., Leng, Y. (2020). Uranium(VI) adsorption on montmorillonite colloid. Journal of Radioanalytical and Nuclear Chemistry, 324 (2), 541–549. doi: https://doi.org/10.1007/s10967-020-07083-y
- Vanhorn, J., Huang, H. (2006). Uranium(VI) bio-coordination chemistry from biochemical, solution and protein structural data. Coordination Chemistry Reviews, 250 (7-8), 765–775. doi: https://doi.org/10.1016/j.ccr.2005.09.010
- Kornilovych, B. Yu., Sorokin, O. H., Pavlenko, V. M., Koshyk, Yu. Y. (2011). Pryrodookhoronni tekhnolohiyi v uranovydobuvniy ta pererobniy promyslovosti. Kyiv: Norma, 156.
- Li, S., Wang, X., Huang, Z., Du, L., Tan, Z., Fu, Y., Wang, X. (2015). Sorption and desorption of uranium(VI) on GMZ bentonite: effect of pH, ionic strength, foreign ions and humic substances. Journal of Radioanalytical and Nuclear Chemistry, 308 (3), 877–886. doi: https://doi.org/10.1007/s10967-015-4513-7
- Yu, T., Chen, Y., Zhang, Y., Tan, X., Xie, T., Shao, B., Huang, X. (2021). Novel reusable sulfate-type zirconium alginate ion-exchanger for fluoride removal. Chinese Chemical Letters, 32 (11), 3410–3415. doi: https://doi.org/10.1016/j.cclet.2021.04.057
- Yan, T., Luo, X., Zou, Z., Lin, X., He, Y. (2017). Adsorption of Uranium(VI) from a Simulated Saline Solution by Alkali-Activated Leather Waste. Industrial & Engineering Chemistry Research, 56 (12), 3251–3258. doi: https://doi.org/10.1021/acs.iecr.6b04425
- Fox, P. M., Davis, J. A., Zachara, J. M. (2006). The effect of calcium on aqueous uranium(VI) speciation and adsorption to ferrihydrite and quartz. Geochimica et Cosmochimica Acta, 70 (6), 1379–1387. doi: https://doi.org/10.1016/j.gca.2005.11.027
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2023 Ihor Pylypenko, Iryna Kovalchuk, Mykola Tsyba

This work is licensed under a Creative Commons Attribution 4.0 International License.
The consolidation and conditions for the transfer of copyright (identification of authorship) is carried out in the License Agreement. In particular, the authors reserve the right to the authorship of their manuscript and transfer the first publication of this work to the journal under the terms of the Creative Commons CC BY license. At the same time, they have the right to conclude on their own additional agreements concerning the non-exclusive distribution of the work in the form in which it was published by this journal, but provided that the link to the first publication of the article in this journal is preserved.
A license agreement is a document in which the author warrants that he/she owns all copyright for the work (manuscript, article, etc.).
The authors, signing the License Agreement with TECHNOLOGY CENTER PC, have all rights to the further use of their work, provided that they link to our edition in which the work was published.
According to the terms of the License Agreement, the Publisher TECHNOLOGY CENTER PC does not take away your copyrights and receives permission from the authors to use and dissemination of the publication through the world's scientific resources (own electronic resources, scientometric databases, repositories, libraries, etc.).
In the absence of a signed License Agreement or in the absence of this agreement of identifiers allowing to identify the identity of the author, the editors have no right to work with the manuscript.
It is important to remember that there is another type of agreement between authors and publishers – when copyright is transferred from the authors to the publisher. In this case, the authors lose ownership of their work and may not use it in any way.








