Identifying the regularities of n-heptane flame inhibition by inorganic compounds
DOI:
https://doi.org/10.15587/1729-4061.2024.301322Keywords:
fire extinguishing agents, n-heptane flame, flame inhibition, flame active radicals, flame temperatureAbstract
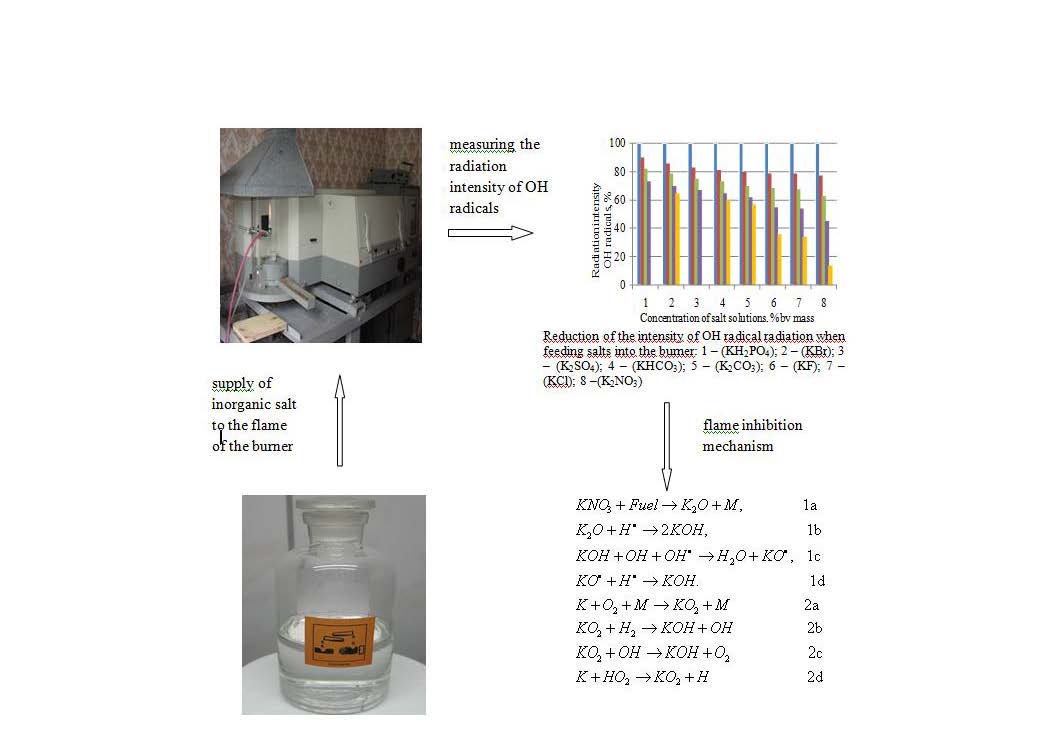
The task related to using inorganic compounds for extinguishing flames is to enable their inhibitory capacity during operation within wide limits. Therefore, the object of the current research was aqueous solutions of inorganic salts, on which the effectiveness of inhibitory properties during interaction with n-heptane flame was established. It has been proven that increasing the mass flow rate of water by 1.5 mg/s reduces the intensity of OH-radicals radiation from 70 % to 30 % and lowers the flame temperature by 90 °C. However, it was found that when potassium salts are given, the intensity of OH-radicals radiation decreases by more than 6 times, potassium chloride and sulfate reduce the intensity of OH-radicals radiation by more than 2.8 times. Among ammonium salts, salts of dihydrogen phosphate and ammonium hydrogen phosphate reduce the relative intensity of radiation of OH radicals by more than 1.3 times. Sodium salts include nitrates and sodium chloride, which reduce the relative radiation intensity of OH radicals by more than 1.6 times. This is manifested, first of all, in the enrichment of the combustible environment with fuel. When determining the flame temperature of flammable liquids, it was established that n-heptane has the most stable and highest flame temperature, which is 1768 °C. When adding inorganic compounds to the flame of n-heptane, nitrate salt, and potassium chloride, the flame temperature increased by less than 20 °C. However, ammonium salts increased the flame temperature to over 140 °C, despite the presence of water. The practical significance is that the results were taken into account during the design and development of extinguishing agents for extinguishing fires. Therefore, there are reasons to assert the possibility of regulating flame extinguishing processes by using inorganic compounds capable of inhibiting active flame radicals
References
- Hao, J., Du, Z., Zhang, T., Li, H. (2022). Influence of NH4H2PO4 powder on the laminar burning velocity of premixed CH4/Air flames. International Journal of Hydrogen Energy, 47 (90), 38477–38493. https://doi.org/10.1016/j.ijhydene.2022.09.003
- Wang, Y., He, J., Yang, J., Lin, C., Ji, W. (2022). Inhibition of polyethylene dust explosion by oxalate and bicarbonate. Huagong Xuebao/CIESC Journal, 73 (9), 4207–4216. https://doi.org/10.11949/0438-1157.20220790
- Wang, Z., Meng, X., Yan, K., Ma, X., Xiao, Q., Wang, J., Bai, J. (2020). Inhibition effects of Al(OH)3 and Mg(OH)2 on Al-Mg alloy dust explosion. Journal of Loss Prevention in the Process Industries, 66, 104206. https://doi.org/10.1016/j.jlp.2020.104206
- Wang, L., Jiang, Y., Qiu, R. (2021). Experimental study of combustion inhibition by trimethyl phosphate in turbulent premixed methane/air flames using OH-PLIF. Fuel, 294, 120324. https://doi.org/10.1016/j.fuel.2021.120324
- Li, W., Jiang, Y., Jin, Y., Wang, L., Xu, W. (2019). Experimental study of the influence of dimethyl methylphosphonate on methane/air coflow diffusion flames using OH-PLIF. Fuel, 235, 39–44. https://doi.org/10.1016/j.fuel.2018.07.088
- Chi, K., Wang, J., Ma, L., Wang, J., Zhou, C. (2020). Synergistic Inhibitory Effect of Free Radical Scavenger/Inorganic Salt Compound Inhibitor on Spontaneous Combustion of Coal. Combustion Science and Technology, 194 (10), 2146–2162. https://doi.org/10.1080/00102202.2020.1858290
- Babushok, V. (1998). Chemical limits to flame inhibition. Combustion and Flame, 115 (4), 551–560. https://doi.org/10.1016/s0010-2180(98)00019-4
- Omar, D., Jaravel, T., Vermorel, O. (2022). On the controlling parameters of the thermal decomposition of inhibiting particles: A theoretical and numerical study. Combustion and Flame, 240, 111991. https://doi.org/10.1016/j.combustflame.2022.111991
- Linteris, G. T., Rumminger, M. D., Babushok, V. I. (2008). Catalytic inhibition of laminar flames by transition metal compounds. Progress in Energy and Combustion Science, 34 (3), 288–329. https://doi.org/10.1016/j.pecs.2007.08.002
- Feng, M.-H., Tao, J.-J., Qin, J., Fei, Q. (2015). Extinguishment of counter-flow diffusion flame by water mist derived from aqueous solutions containing chemical additives. Journal of Fire Sciences, 34 (1), 51–68. https://doi.org/10.1177/0734904115618220
- Badhuk, P., Ravikrishna, R. V. (2022). Development and validation of skeletal/global mechanisms describing TMP-based flame inhibition. Combustion Theory and Modelling, 26 (5), 968–987. https://doi.org/10.1080/13647830.2022.2090443
- Likhnyovskyi, R., Tsapko, Y., Kovalenko, V., Onyshchuk, A. (2023). The Possibility of Using 1301 and 2402 Mixtures of Halons for Fire Extinguishing Purposes. Key Engineering Materials, 954, 135–144. https://doi.org/10.4028/p-coko1k
- Tsapko, Y., Sokolenko, K., Vasylyshyn, R., Melnyk, O., Tsapko, А., Bondarenko, O., Karpuk, A. (2022). Establishing patterns of nitrogen application for fire safety of sunflower grain storage facilities. Eastern-European Journal of Enterprise Technologies, 5 (10 (119)), 57–65. https://doi.org/10.15587/1729-4061.2022.266014
- Tsapko, Y., Likhnyovskyi, R., Tsapko, А., Kovalenko, V., Slutska, O., Illiuchenko, P. et al. (2023). Determining the patterns of extinguishing polar flammable liquids with a film-forming foaming agent. Eastern-European Journal of Enterprise Technologies, 3 (10 (123)), 48–56. https://doi.org/10.15587/1729-4061.2023.278910
- Tsapko, Y., Rogovskii, I., Titova, L., Bilko, T., Tsapko, А., Bondarenko, O., Mazurchuk, S. (2020). Establishing regularities in the insulating capacity of a foaming agent for localizing flammable liquids. Eastern-European Journal of Enterprise Technologies, 5 (10 (107)), 51–57. https://doi.org/10.15587/1729-4061.2020.215130
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2024 Yuriy Tsapko, Аleksii Tsapko, Ruslan Likhnyovskyi, Maryna Sukhanevych, Oksana Slutska, Natalia Lialina, Olga Bondarenko

This work is licensed under a Creative Commons Attribution 4.0 International License.
The consolidation and conditions for the transfer of copyright (identification of authorship) is carried out in the License Agreement. In particular, the authors reserve the right to the authorship of their manuscript and transfer the first publication of this work to the journal under the terms of the Creative Commons CC BY license. At the same time, they have the right to conclude on their own additional agreements concerning the non-exclusive distribution of the work in the form in which it was published by this journal, but provided that the link to the first publication of the article in this journal is preserved.
A license agreement is a document in which the author warrants that he/she owns all copyright for the work (manuscript, article, etc.).
The authors, signing the License Agreement with TECHNOLOGY CENTER PC, have all rights to the further use of their work, provided that they link to our edition in which the work was published.
According to the terms of the License Agreement, the Publisher TECHNOLOGY CENTER PC does not take away your copyrights and receives permission from the authors to use and dissemination of the publication through the world's scientific resources (own electronic resources, scientometric databases, repositories, libraries, etc.).
In the absence of a signed License Agreement or in the absence of this agreement of identifiers allowing to identify the identity of the author, the editors have no right to work with the manuscript.
It is important to remember that there is another type of agreement between authors and publishers – when copyright is transferred from the authors to the publisher. In this case, the authors lose ownership of their work and may not use it in any way.








