Determination of the possibility of the synthesis of Zn-Al layered double hydroxides, intercalated with peroxyanions, as a perspective solid disinfectant
DOI:
https://doi.org/10.15587/1729-4061.2024.303030Keywords:
Zn–Al double-layer hydroxide, peroxylactic acid, solid disinfectant, intercalation, chemical coprecipitationAbstract
Infectious diseases in the modern world pose a significant threat to humanity in the form of epidemics and pandemics. To prevent and combat them, it is necessary to carry out antiseptic and disinfectant treatments of various environments, household and industrial surfaces, as well as wounds of various origins. Double-layer hydroxides intercalated with peroxyanions as active oxygen compounds are promising materials for this.
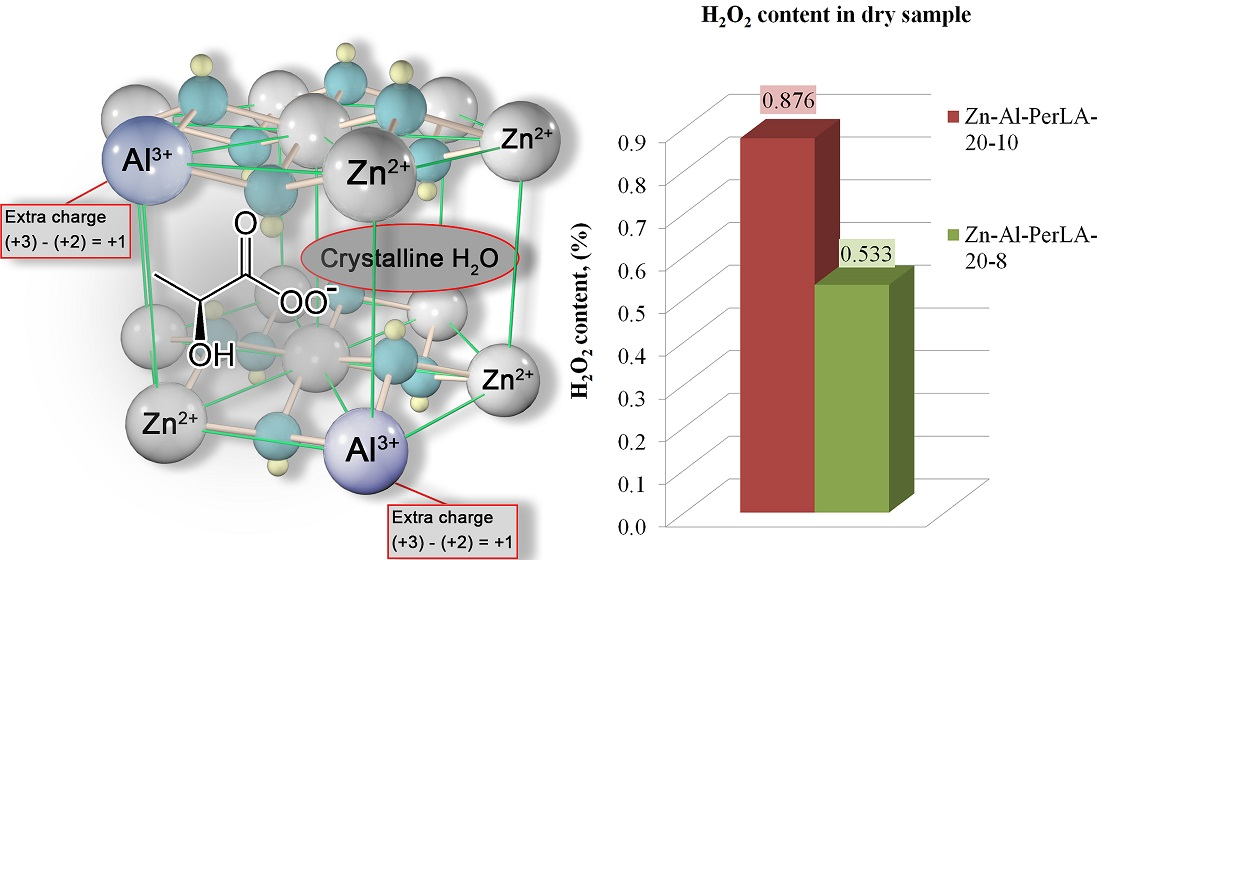
In order to determine the possibility of obtaining Zn-Al double-layer hydroxide intercalated with peroxylactic acid anions, samples were synthesized by the method of chemical co-precipitation in the presence of peroxylactic acid at controlled pH (8, 10) and t=20 ℃. The properties of the synthesized samples were investigated. The content of active oxygen (in terms of H2O2) was determined by the method of iodometric titration with the calculation of the percentage of hydrogen peroxide that was intercalated in double layered hydroxides, remained in the mother solution or was lost. The crystal structure was studied by X-ray phase analysis, the yield of samples was determined gravimetrically, and sedimentation was determined by measuring and normalizing the thickness of the sediment layer.
It was found that the samples synthesized at pH=8 and 10 are biphasic and consist of an oxide phase and a double-layer hydroxide phase. The determined content of active oxygen (in terms of H2O2) in the samples synthesized at pH=8 (0.533 %) and at pH=10 (0.876 %) confirms the success of the synthesis of Zn-Al-peroxylactate double layered hydroxides. Synthesis at elevated pH is promising. A low percentage of H2O2 intercalation was revealed – 4.03–6.54 %, the majority of hydrogen peroxide (82.36-94.44 %) remains in the mother solution.
The yield of the synthesized samples was determined to be 61.9 % and 79.5 % at synthesis pH of 10 and 8, respectively. The sedimentation properties of the samples were studied and their improvement was shown when the pH of the synthesis was increased
References
- McDonnell, G., Russell, A. D. (1999). Antiseptics and Disinfectants: Activity, Action, and Resistance. Clinical Microbiology Reviews, 12 (1), 147–179. https://doi.org/10.1128/cmr.12.1.147
- Kitis, M. (2004). Disinfection of wastewater with peracetic acid: a review. Environment International, 30 (1), 47–55. https://doi.org/10.1016/s0160-4120(03)00147-8
- Vimont, A., Fliss, I., Jean, J. (2014). Study of the Virucidal Potential of Organic Peroxyacids Against Norovirus on Food-Contact Surfaces. Food and Environmental Virology, 7 (1), 49–57. https://doi.org/10.1007/s12560-014-9174-0
- Kovalenko, V., Kotok, V., Murashevych, B. (2023). Layered Double Hydroxides as the Unique Product of Target Ionic Construction for Energy, Chemical, Foods, Cosmetics, Medicine and Ecology Applications. The Chemical Record, 24 (2). https://doi.org/10.1002/tcr.202300260
- Khan, A. I., Ragavan, A., Fong, B., Markland, C., O’Brien, M., Dunbar, T. G. et al. (2009). Recent Developments in the Use of Layered Double Hydroxides as Host Materials for the Storage and Triggered Release of Functional Anions. Industrial & Engineering Chemistry Research, 48 (23), 10196–10205. https://doi.org/10.1021/ie9012612
- Kovalenko, V., Kotok, V. (2019). Influence of the carbonate ion on characteristics of electrochemically synthesized layered (α+β) nickel hydroxide. Eastern-European Journal of Enterprise Technologies, 1 (6 (97)), 40–46. https://doi.org/10.15587/1729-4061.2019.155738
- Kotok, V., Kovalenko, V. (2018). A study of the effect of tungstate ions on the electrochromic properties of Ni(OH)2 films. Eastern-European Journal of Enterprise Technologies, 5 (12 (95)), 18–24. https://doi.org/10.15587/1729-4061.2018.145223
- Bharali, D., Deka, R. C. (2017). Adsorptive removal of congo red from aqueous solution by sonochemically synthesized NiAl layered double hydroxide. Journal of Environmental Chemical Engineering, 5 (2), 2056–2067. https://doi.org/10.1016/j.jece.2017.04.012
- Kovalenko, V., Kotok, V., Yeroshkina, A., Zaychuk, A. (2017). Synthesis and characterisation of dyeintercalated nickelaluminium layereddouble hydroxide as a cosmetic pigment. Eastern-European Journal of Enterprise Technologies, 5 (12 (89)), 27–33. https://doi.org/10.15587/1729-4061.2017.109814
- Darmograi, G., Prelot, B., Layrac, G., Tichit, D., Martin-Gassin, G., Salles, F., Zajac, J. (2015). Study of Adsorption and Intercalation of Orange-Type Dyes into Mg–Al Layered Double Hydroxide. The Journal of Physical Chemistry C, 119 (41), 23388–23397. https://doi.org/10.1021/acs.jpcc.5b05510
- Tian, Y., Wang, G., Li, F., Evans, D. G. (2007). Synthesis and thermo-optical stability of o-methyl red-intercalated Ni–Fe layered double hydroxide material. Materials Letters, 61 (8-9), 1662–1666. https://doi.org/10.1016/j.matlet.2006.07.094
- Nalawade, P., Aware, B., Kadam, V. J., Hirlekar, R. S. (2009). Layered double hydroxides: A review. Journal of Scientific & Industrial Research, 68 (4), 267–272.
- Delhoyo, C. (2007). Layered double hydroxides and human health: An overvie. Applied Clay Science, 36 (1-3), 103–121. https://doi.org/10.1016/j.clay.2006.06.010
- Demkina, E. V., Ilicheva, E. A., El-Registan, G. I., Pankratov, T. A., Yushina, Y. K., Semenova, A. A., Nikolaev, Y. A. (2023). New Approach to Improving the Efficiency of Disinfectants against Biofilms. Coatings, 13 (3), 582. https://doi.org/10.3390/coatings13030582
- Rüsch gen. Klaas, M., Steffens, K., Patett, N. (2002). Biocatalytic peroxy acid formation for disinfection. Journal of Molecular Catalysis B: Enzymatic, 19-20, 499–505. https://doi.org/10.1016/s1381-1177(02)00204-7
- Hu, M., Lei, L. (2006). Effects of particle size on the electrochemical performances of a layered double hydroxide, [Ni4Al(OH)10]NO3. Journal of Solid State Electrochemistry, 11 (6), 847–852. https://doi.org/10.1007/s10008-006-0231-y
- Solovov, V. A., Nikolenko, N. V., Kovalenko, V. L., Kotok, V. A., Burkov, A. А., Kondrat’ev, D. A. et. al. (2018). Synthesis of Ni(II)-Ti(IV) Layered Double Hydroxides Using Coprecipitation At High Supersaturation Method. ARPN Journal of Engineering and Applied Sciences, 13 (24), 9652–9656.
- Xiao-yan, G., Jian-cheng, D. (2007). Preparation and electrochemical performance of nano-scale nickel hydroxide with different shapes. Materials Letters, 61 (3), 621–625. https://doi.org/10.1016/j.matlet.2006.05.026
- Saikia, H., Ganguli, J. N. (2012). Intercalation of Azo Dyes in Ni-Al Layered Double Hydroxides. Asian Journal of Chemistry, 24 (12), 5909–5913.
- Kotok, V. A., Kovalenko, V. L., Solovov, V. A., Kovalenko, P. V., Ananchenko, B. A. (2018). Effect of deposition time on properties of electrochromic nickel hydroxide films prepared by cathodic template synthesis. ARPN Journal of Engineering and Applied Sciences, 13 (9), 3076–3086.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2024 Vadym Kovalenko, Anastasiia Borysenko, Valerii Kotok, Volodymyr Verbitskiy, Volodymyr Medianyk, Viktoriia Stoliarenko, Yuriy Pepa, Serhii Simchenko, Viktor Ved, Sheikh Ahmad Izaddin Sheikh Mohd Ghazali

This work is licensed under a Creative Commons Attribution 4.0 International License.
The consolidation and conditions for the transfer of copyright (identification of authorship) is carried out in the License Agreement. In particular, the authors reserve the right to the authorship of their manuscript and transfer the first publication of this work to the journal under the terms of the Creative Commons CC BY license. At the same time, they have the right to conclude on their own additional agreements concerning the non-exclusive distribution of the work in the form in which it was published by this journal, but provided that the link to the first publication of the article in this journal is preserved.
A license agreement is a document in which the author warrants that he/she owns all copyright for the work (manuscript, article, etc.).
The authors, signing the License Agreement with TECHNOLOGY CENTER PC, have all rights to the further use of their work, provided that they link to our edition in which the work was published.
According to the terms of the License Agreement, the Publisher TECHNOLOGY CENTER PC does not take away your copyrights and receives permission from the authors to use and dissemination of the publication through the world's scientific resources (own electronic resources, scientometric databases, repositories, libraries, etc.).
In the absence of a signed License Agreement or in the absence of this agreement of identifiers allowing to identify the identity of the author, the editors have no right to work with the manuscript.
It is important to remember that there is another type of agreement between authors and publishers – when copyright is transferred from the authors to the publisher. In this case, the authors lose ownership of their work and may not use it in any way.








