Choosing an efficient mass exchange apparatus for desorption of hydrogen sulphide from reservoir and drainage waters
DOI:
https://doi.org/10.15587/1729-4061.2024.314049Keywords:
hydrogen sulfide, water purification, mass exchange devices, desorption rate, bubbling, solution dispersionAbstract
Industrial wastewater is often contaminated with hydrogen sulfide and sulfides. This poses significant risks to both the environment and human health and life as H2S is extremely toxic. Therefore, water purification from it is vital, and the choice of an effective desorber device is an urgent issue.
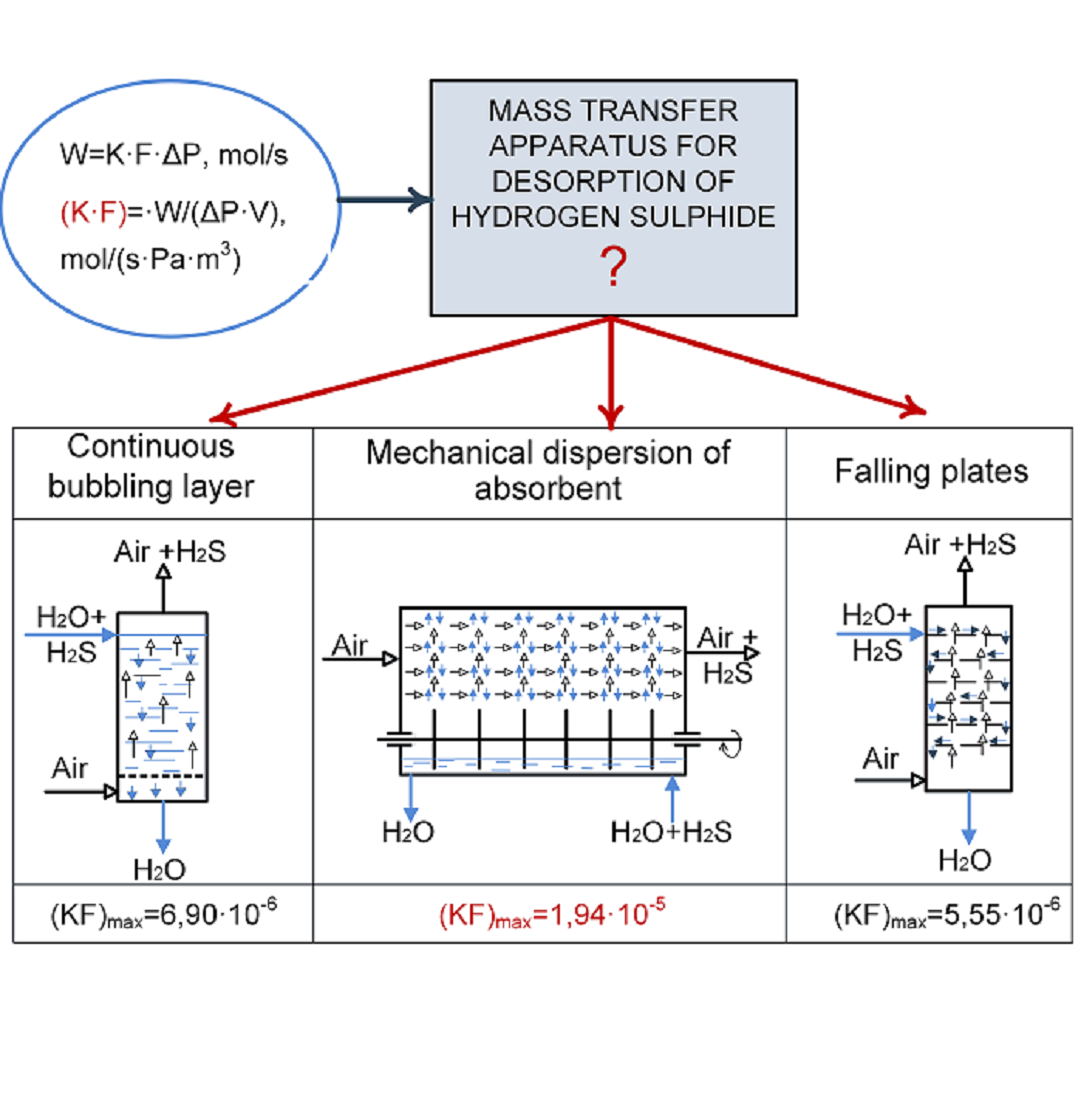
This paper investigates the process of H2S desorption from wastewater in mass exchange devices with a continuous bubbling bed (DCBB), a column with falling plates (CFP), and a horizontal device with bucket-like dispersers (HDBD). To analyze the kinetic and technological characteristics of the process, the following indicators were selected: the product of the mass exchange coefficient on the contact surface of phases (K·F), reduced to 1 m3 of the volume of the apparatus, and the degree of hydrogen sulfide desorption.
The most complete desorption of hydrogen sulfide occurs at pH≤5. For practical needs, it is suggested to acidify the water to pH=5.5...6.0. It was established that the partial pressure of H2S increases linearly with increasing temperature, and an increase in salinity from 2...4 to 130...160 kg/m3 leads to its increase by 1.45...1.5 times.
The best desorption indicators can be achieved in HDBD at pH=4.97. The efficiency of cleaning in CFP and DCBB is significantly affected by the specific air flow rate. The highest values (K·F) per 1 m3 that were achieved in desorbers are, mol/(s·Pa·m3): HDBD – 1.94·10-5, in CFP – 5.55·10-6, DCBB – 6.9·10-6. The ratio of the product (K·F) in HDBD to CFP is 3.5, and in HDBD to DCBB 2.8. It was possible to achieve the maximum degree of desorption of 37.8 % in DCBB; in CFP, this indicator is 74.1 %, and in HDBD – 77.7 %. Experimental studies have generally confirmed the effectiveness of using HDBD, and the results obtained under production conditions on real drainage and reservoir waters could find be practically implemented in hydrogen sulfide utilization technologies
References
- Mokhatab, S., Poe, W. A., Mak, J. Y. (2019). Sulfur Recovery and Handling. Handbook of Natural Gas Transmission and Processing, 271–305. https://doi.org/10.1016/b978-0-12-815817-3.00008-3
- Latha, A., Arivukarasi, M. C., Keerthana, C. M., Subashri, R., Vishnu Priya, V. (2018). Paper and Pulp Industry Manufacturing and Treatment Processes A Review. International Journal of Engineering Research And, V6 (02). https://doi.org/10.17577/ijertcon011
- Hydrogen Sulfide. Hazards. Occupational Safety and Health Administration. Available at: https://www.osha.gov/hydrogen-sulfide/hazards
- Simonton, D. S. (2014). Hydrogen Sulfide Exposure and Human-Health Risk in Mining-Impacted Regions. World Environmental and Water Resources Congress 2014, 1001–1009. https://doi.org/10.1061/9780784413548.100
- Pudi, A., Rezaei, M., Signorini, V., Andersson, M. P., Baschetti, M. G., Mansouri, S. S. (2022). Hydrogen sulfide capture and removal technologies: A comprehensive review of recent developments and emerging trends. Separation and Purification Technology, 298, 121448. https://doi.org/10.1016/j.seppur.2022.121448
- Reverberi, A. P., Klemeš, J. J., Varbanov, P. S., Fabiano, B. (2016). A review on hydrogen production from hydrogen sulphide by chemical and photochemical methods. Journal of Cleaner Production, 136, 72–80. https://doi.org/10.1016/j.jclepro.2016.04.139
- Mulewa, W., Tahir, M. (2024). Perspectives in the Synergetic Photothermocatalysis of Hydrogen Sulfide Decomposition for Hydrogen Production: A Comprehensive Review. Energy & Fuels, 38 (17), 15972–15997. https://doi.org/10.1021/acs.energyfuels.4c02471
- Yavorskyi, V., Helesh, A., Yavorskyi, I., Kalymon, Y. (2016). А theoretical analysis of chemisorption of sulfur (IV) oxide. Rationale for the choice of an efficient mass-exchange apparatus. Eastern-European Journal of Enterprise Technologies, 1 (6 (79)), 32–40. https://doi.org/10.15587/1729-4061.2016.60312
- Ntagia, E., Prévoteau, A., Rabaey, K. (2020). Electrochemical removal of sulfur pollution. Environmental Technologies to Treat Sulphur Pollution: Principles and Engineering, 247–276. https://doi.org/10.2166/9781789060966_0247
- Enache, A.-F., Dan, M. L., Vaszilcsin, N. (2018). Electrochemical Oxidation of Sulphite in Neutral Media on Platinum Anode. International Journal of Electrochemical Science, 13 (5), 4466–4478. https://doi.org/10.20964/2018.05.07
- Qi, R., Lin, T., Sheng, K., Lin, H. (2024). Insight into the effective electrocatalytic sulfide removal from aqueous solutions using surface oxidized stainless-steel anode and its desulfurization mechanism. Science of The Total Environment, 931, 172570. https://doi.org/10.1016/j.scitotenv.2024.172570
- Wei, J., Wu, X. (2024). The potential of coupled water electrolysis with electrochemical wastewater treatments. International Journal of Hydrogen Energy, 68, 745–754. https://doi.org/10.1016/j.ijhydene.2024.04.308
- Wei, J., Wu, X. (2024). Electrochemical processes for simultaneous sulfur and energy recoveries from sulfide-containing wastewater. Separation and Purification Technology, 348, 127621. https://doi.org/10.1016/j.seppur.2024.127621
- Wu, S., Zhang, L., Sun, B., Zou, H., Zeng, X., Luo, Y. et al. (2017). Mass-Transfer Performance for CO2 Absorption by 2-(2-Aminoethylamino)ethanol Solution in a Rotating Packed Bed. Energy & Fuels, 31 (12), 14053–14059. https://doi.org/10.1021/acs.energyfuels.7b03002
- Yavorskiy, V., Helesh, A. (2015). Theoretical Analysis of Efficiency of Horizontal Apparatus with Bucket-like Dispersers in the Dust Trapping System. Chemistry & Chemical Technology, 9 (4), 471–478. https://doi.org/10.23939/chcht09.04.471
- Yavorskyi, V., Helesh, A., Yavorskyi, I. (2013). Principals for the Creation of Effective and Economically Sound Treating Processes of Industrial Emissions with Sulfur Oxide Low Content. Chemistry & Chemical Technology, 7 (2), 205–211. https://doi.org/10.23939/chcht07.02.205
- Yavorskiy, V., Helesh, A. (2016). Waste Gases Cleaning at the Production of Ferrum Oxide Pigment Using Horizontal Apparatus with Bucket-like Dispersers. Chemistry & Chemical Technology, 10 (2), 193–199. https://doi.org/10.23939/chcht10.02.193
- Helesh, A., Yavorskyi, V., Yavorskyi, I. (2016). Chemisorption of sulfur (IV) oxide using the horizontal apparatus with bucket-like dispersers. Eastern-European Journal of Enterprise Technologies, 2 (6 (80)), 46–52. https://doi.org/10.15587/1729-4061.2016.63956
- Kalymon, Ya. A., Helesh, A. B., Slyuzar, A. V., Znak, Z. O. (2022). Theoretical studies of H2S, SO2 and O2 absorption in mass exchanged apparatus with a continuous bubbling layer and mechanical dispersion of an absorbent. Voprosy Khimii i Khimicheskoi Tekhnologii, 3, 33–43. https://doi.org/10.32434/0321-4095-2022-142-3-33-43
- Peng, C., Mao, S., Hu, J., He, L. (2019). A Helmholtz free energy equation of state for the vapor-liquid equilibrium and PVTx properties of the H2S H2O mixture and its application to the H2S H2O NaCl system. Applied Geochemistry, 101, 19–30. https://doi.org/10.1016/j.apgeochem.2018.12.021
- Tari, F., Shekarriz, M., Zarrinpashne, S., Ruzbehani, A. (2018). Investigation on Solubility of Hydrogen Sulfide in Molten Sulfur Using Iodometric Back Titration Method. Journal of Gas Technology, 3 (1), 14–20. Available at: https://dorl.net/dor/20.1001.1.25885596.2018.3.1.2.8
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2024 Andriy Helesh, Yaroslav Kalymon

This work is licensed under a Creative Commons Attribution 4.0 International License.
The consolidation and conditions for the transfer of copyright (identification of authorship) is carried out in the License Agreement. In particular, the authors reserve the right to the authorship of their manuscript and transfer the first publication of this work to the journal under the terms of the Creative Commons CC BY license. At the same time, they have the right to conclude on their own additional agreements concerning the non-exclusive distribution of the work in the form in which it was published by this journal, but provided that the link to the first publication of the article in this journal is preserved.
A license agreement is a document in which the author warrants that he/she owns all copyright for the work (manuscript, article, etc.).
The authors, signing the License Agreement with TECHNOLOGY CENTER PC, have all rights to the further use of their work, provided that they link to our edition in which the work was published.
According to the terms of the License Agreement, the Publisher TECHNOLOGY CENTER PC does not take away your copyrights and receives permission from the authors to use and dissemination of the publication through the world's scientific resources (own electronic resources, scientometric databases, repositories, libraries, etc.).
In the absence of a signed License Agreement or in the absence of this agreement of identifiers allowing to identify the identity of the author, the editors have no right to work with the manuscript.
It is important to remember that there is another type of agreement between authors and publishers – when copyright is transferred from the authors to the publisher. In this case, the authors lose ownership of their work and may not use it in any way.








