Identifying the effect of varying acid concentration and solid/liquid ratio in the leaching extraction of magnesium from ferronickel slag
DOI:
https://doi.org/10.15587/1729-4061.2024.319045Keywords:
ferronickel slag, recycling, alkali fusion, roasting, water leaching, acid leaching, extraction, magnesium, acid concentration, solid/liquid ratioAbstract
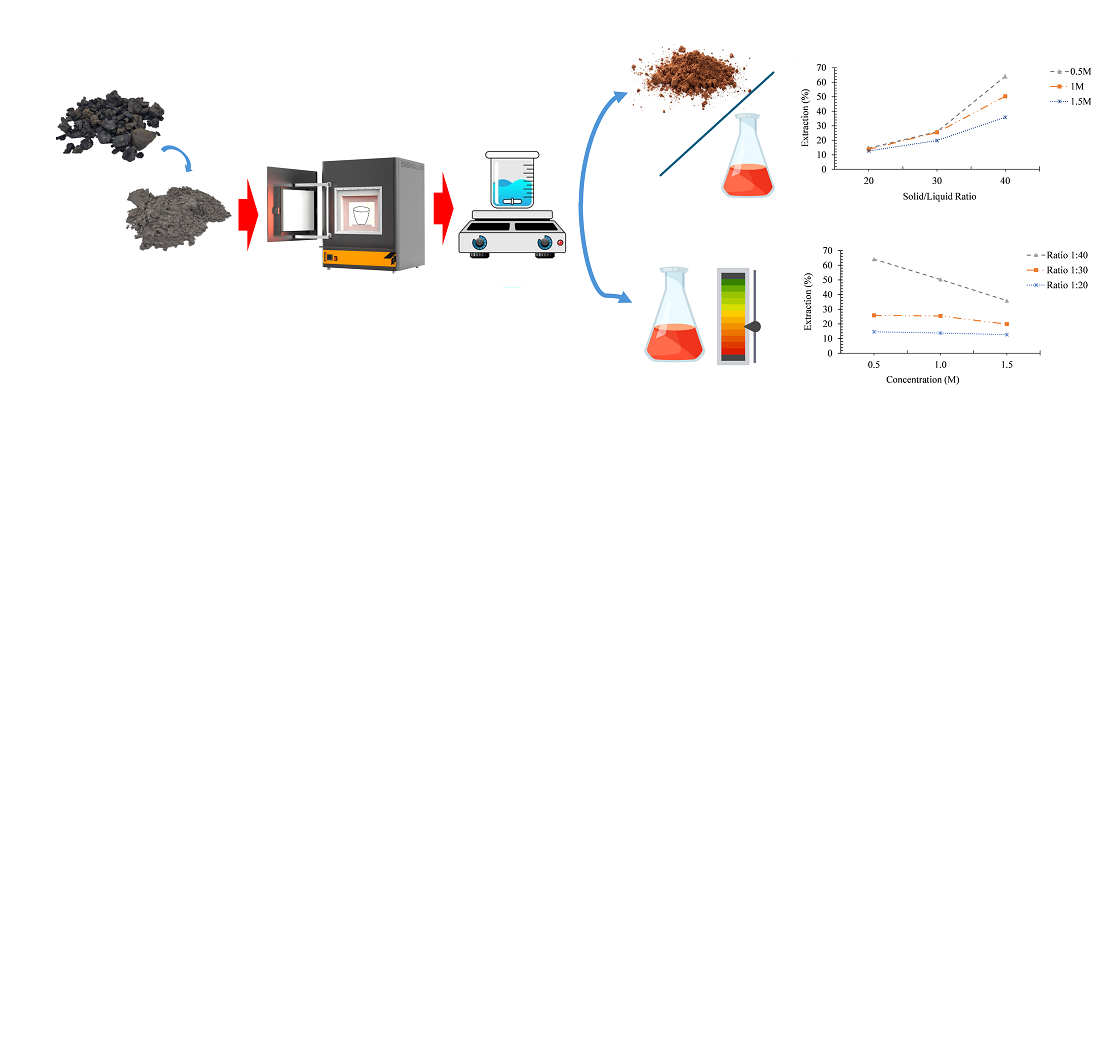
Indonesia is the country with the largest nickel reserves and production levels in the world. Each ton of nickel production can produce eight tons of by-products in the form of ferronickel slag, which continues to increase due to the minimal recycling process of these by-products. This study aimed to determine the impact of changes in acid concentration and solid/liquid ratio on the leaching extraction of magnesium from ferronickel slag and characterize the transformation of ferronickel slag at each stage of the process. The research was conducted using the alkali fusion method and continued with water leaching and acid leaching using Hydrochloric Acid (HCl) as the solvent. The first step in the investigation was milling to get a powder with the particle size ≤127 µm. The sample powder was subsequently mixed with the Na2CO3 additive in a 50:50 (w/w) ratio and roasted for 60 minutes at 1000 °C. The water leaching procedure was then conducted for 60 minutes at 100 °C, a 1:10 (w/v) ratio, and a stirring speed of 400 rpm. The filtrate and residue were then separated using a filtration process. An acid leaching utilizing HCl with concentration variations of 0.5, 1, and 1.5 M, and solid/liquid ratio (s/l) variations of 1:20, 1:30, and 1:40 (w/v) was performed on the residue. The results of acid leaching were then filtrated again. The filtrate was then characterized by ICP-OES testing. Based on the analysis results, it can be stated that the percentage of magnesium extraction increases as the solid/liquid ratio (s/l) increases but decreases with the increase in HCl concentration. The optimum percentage of magnesium extraction is 64.12 %, which was achieved with the leaching conditions of a solid/liquid ratio (s/l) of 1:40 (w/v) and a HCl concentration of 0.5 M
References
- Nickel Statistics and Information. USGS. Available at: https://www.usgs.gov/centers/national-minerals-information-center/nickel-statistics-and-information
- Nurjaman, F., Astuti, W., Bahfie, F., Suharno, B. (2021). Study of selective reduction in lateritic nickel ore: Saprolite versus limonite. Materials Today: Proceedings, 44, 1488–1494. https://doi.org/10.1016/j.matpr.2020.11.687
- Sun, W., Li, X., Liu, R., Zhai, Q., Li, J. (2021). Recovery of Valuable Metals from Nickel Smelting Slag Based on Reduction and Sulfurization Modification. Minerals, 11 (9), 1022. https://doi.org/10.3390/min11091022
- Ulum, R. M., Natalin, Riastuti, R., Mayangsari, W., Prasetyo, A. B., Soedarsono, J. W., Maksum, A. (2023). Pyro-Hydrometallurgy Routes to Recover Silica from Indonesian Ferronickel Slag. Recycling, 8 (1), 13. https://doi.org/10.3390/recycling8010013
- Zulhan, Z., Agustina, N. (2021). A novel utilization of ferronickel slag as a source of magnesium metal and ferroalloy production. Journal of Cleaner Production, 292, 125307. https://doi.org/10.1016/j.jclepro.2020.125307
- Prasetyo, A. B., Khaerul, A., Mayangsari, W., Febriana, E., Maksum, A., Andinie, J. et al. (2021). Magnesium extraction of ferronickel slag processed by alkali fusion and hydrochloric acid leaching. Journal of Mining and Metallurgy, Section B: Metallurgy, 57 (2), 225–233. https://doi.org/10.2298/jmmb200224018p
- Yan, P., Shen, Y., Du, X., Chong, J. (2020). Microwave Absorption Properties of Magnetite Particles Extracted from Nickel Slag. Materials, 13 (9), 2162. https://doi.org/10.3390/ma13092162
- Li, B., Rong, T., Du, X., Shen, Y., Shen, Y. (2021). Preparation of Fe3O4 particles with unique structures from nickel slag for enhancing microwave absorption properties. Ceramics International, 47 (13), 18848–18857. https://doi.org/10.1016/j.ceramint.2021.03.224
- Gao, F., Huang, Z., Li, H., Li, X., Wang, K., Hamza, M. F. et al. (2021). Recovery of magnesium from ferronickel slag to prepare hydrated magnesium sulfate by hydrometallurgy method. Journal of Cleaner Production, 303, 127049. https://doi.org/10.1016/j.jclepro.2021.127049
- Gu, F., Peng, Z., Tang, H., Ye, L., Tian, W., Liang, G. et al. (2018). Preparation of Refractory Materials from Ferronickel Slag. Characterization of Minerals, Metals, and Materials 2018, 633–642. https://doi.org/10.1007/978-3-319-72484-3_67
- Abdul, F., Adachi, K., Ho, H.-J., Iizuka, A., Shibata, E. (2024). Magnesium recovery from ferronickel slag by reaction with sodium hydroxide. Journal of Environmental Chemical Engineering, 12 (3), 112516. https://doi.org/10.1016/j.jece.2024.112516
- Song, H.-Y., Seo, J.-B., Kang, S.-K., Kim, I.-D., Choi, B.-W., Oh, K.-J. (2014). CO2Fixation by Magnesium Hydroxide from Ferro-Nickel Slag. Clean Technology, 20 (1), 42–50. https://doi.org/10.7464/ksct.2014.20.1.042
- Mubarok, M. Z., Yudiarto, A. (2017). Synthesis of Magnesium Oxide from Ferronickel Smelting Slag Through Hydrochloric Acid Leaching-Precipitation and Calcination. Energy Technology 2017, 247–258. https://doi.org/10.1007/978-3-319-52192-3_24
- Yang, J., Duan, X., Liu, L., Yang, H., Jiang, X. (2021). Recovery of Magnesium from Ferronickel Slag to Prepare Magnesium Oxide by Sulfuric Acid Leaching. Minerals, 11 (12), 1375. https://doi.org/10.3390/min11121375
- Pangaribuan, R. H., Patrick, J., Prasetyo, A. B., Maksum, A., Munir, B., Soedarsono, J. W. (2018). The effect of NaOH (natrium hydroxide) to slag nickel pyrometallurgy in different temperature and additive ratio. E3S Web of Conferences, 67, 03052. https://doi.org/10.1051/e3sconf/20186703052
- Patrick, J., Prasetyo, A. B., Munir, B., Maksum, A., Soedarsono, J. W. (2018). The effect of addition of sodium sulphate (Na2SO4) to nickel slag pyrometallurgical process with temperature and additives ratio as variables. E3S Web of Conferences, 67, 03053. https://doi.org/10.1051/e3sconf/20186703053
- Mufakhir, F. R., Mubarok, M. Z., Ichlas, Z. T. (2018). Leaching of silicon from ferronickel (FeNi) smelting slag with sodium hydroxide solution at atmospheric pressure. IOP Conference Series: Materials Science and Engineering, 285, 012003. https://doi.org/10.1088/1757-899x/285/1/012003
- Prasetyo, A. B., Darmawansyah, R., Mayangsari, W., Febriana, E., Permana, S., Maksum, A. et al. (2020). Reverse leaching of magnesium from ferronickel slag using alkali solvent NaOH. Eastern-European Journal of Enterprise Technologies, 1 (12 (103)), 6–14. https://doi.org/10.15587/1729-4061.2020.193885
- Xiao, Q., Chen, Y., Gao, Y., Xu, H., Zhang, Y. (2010). Leaching of silica from vanadium-bearing steel slag in sodium hydroxide solution. Hydrometallurgy, 104 (2), 216–221. https://doi.org/10.1016/j.hydromet.2010.06.007
- Qian, B., Liu, H., Ma, B., Wang, Q., Lu, J., Hu, Y. et al. (2022). Bulk trash to nano treasure: Synthesis of two-dimensional brucite nanosheet from high-magnesium nickel slag. Journal of Cleaner Production, 333, 130196. https://doi.org/10.1016/j.jclepro.2021.130196
- Mayangsari, W., Avifah, I. N., Prasetyo, A. B., Febriana, E., Maksum, A., Ulum, R. M. et al. (2021). Decomposition of ferronickel slag through alkali fusion in the roasting process. Eastern-European Journal of Enterprise Technologies, 2 (12 (110)), 44–51. https://doi.org/10.15587/1729-4061.2021.217579
- Prasetyo, A. B., Maksum, A., Soedarsono, J. W., Firdiyono, F. (2019). Thermal characteristics of ferronickel slag on roasting process with addition of sodium carbonate (Na2CO3). IOP Conference Series: Materials Science and Engineering, 541 (1), 012037. https://doi.org/10.1088/1757-899x/541/1/012037
- Raschman, P., Špáková, M., Fedoročková, A. (2010). Effect of hydrochloric acid concentration on the selectivity of leaching of high-calcium dead-burned magnesite. Acta Montanistica Slovaca, 15 (3), 232–237. Available at: https://actamont.tuke.sk/pdf/2010/n3/09_Raschman.pdf
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2024 Eni Febriana, Aldo Yansen Tambor Napitupulu, Johny Wahyuadi Soedarsono, Badrul Munir, Eddy Sumarno Siradj, Agus Paul Setiawan Kaban, Martina Yttria Mertoprawiro, Kadek Ambara Jaya

This work is licensed under a Creative Commons Attribution 4.0 International License.
The consolidation and conditions for the transfer of copyright (identification of authorship) is carried out in the License Agreement. In particular, the authors reserve the right to the authorship of their manuscript and transfer the first publication of this work to the journal under the terms of the Creative Commons CC BY license. At the same time, they have the right to conclude on their own additional agreements concerning the non-exclusive distribution of the work in the form in which it was published by this journal, but provided that the link to the first publication of the article in this journal is preserved.
A license agreement is a document in which the author warrants that he/she owns all copyright for the work (manuscript, article, etc.).
The authors, signing the License Agreement with TECHNOLOGY CENTER PC, have all rights to the further use of their work, provided that they link to our edition in which the work was published.
According to the terms of the License Agreement, the Publisher TECHNOLOGY CENTER PC does not take away your copyrights and receives permission from the authors to use and dissemination of the publication through the world's scientific resources (own electronic resources, scientometric databases, repositories, libraries, etc.).
In the absence of a signed License Agreement or in the absence of this agreement of identifiers allowing to identify the identity of the author, the editors have no right to work with the manuscript.
It is important to remember that there is another type of agreement between authors and publishers – when copyright is transferred from the authors to the publisher. In this case, the authors lose ownership of their work and may not use it in any way.








