Electrolysis and precipitation-based purification of ferronickel for high-purity nickel production
DOI:
https://doi.org/10.15587/1729-4061.2025.324608Keywords:
ferronickel, secondary, resources, local, content, electrolysis, precipitation, pH, temperature, timeAbstract
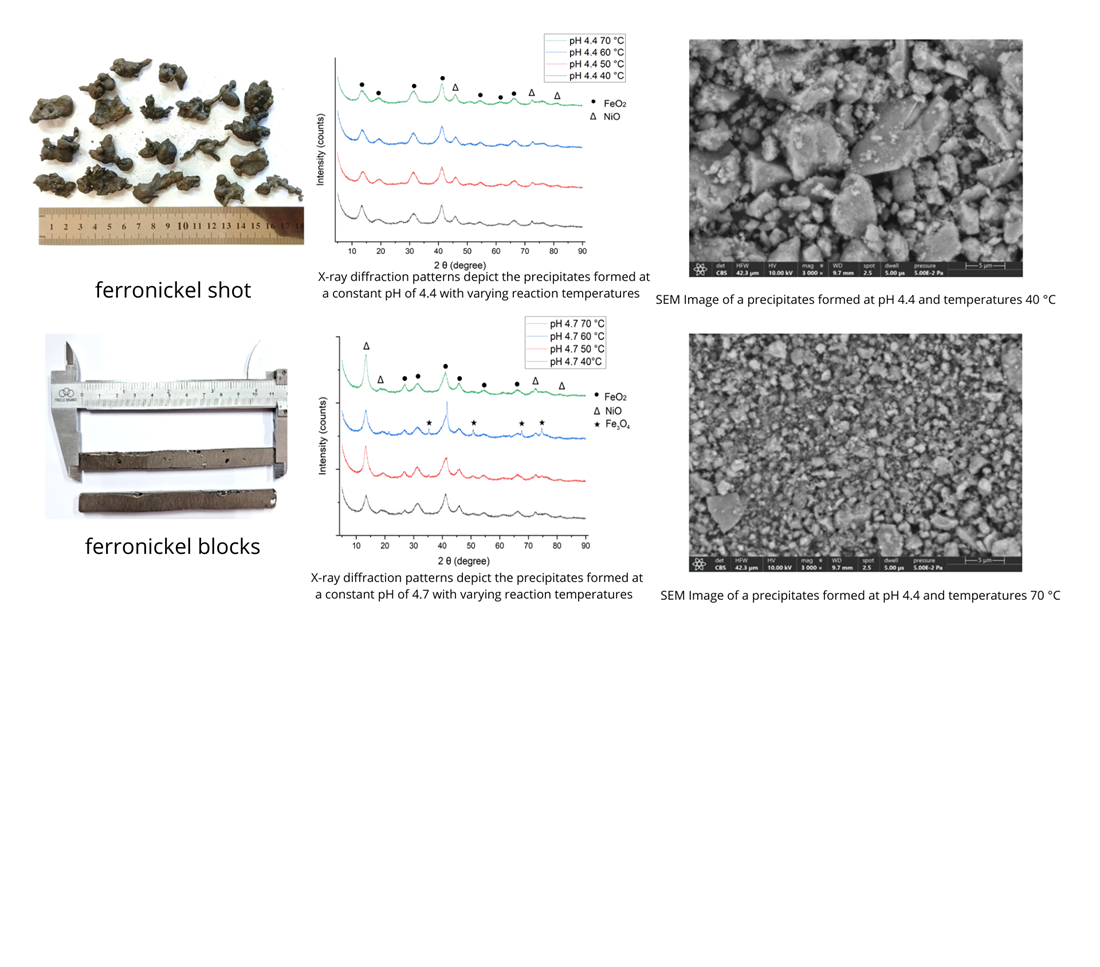
This study investigates the purification of ferronickel through electrolysis and precipitation processes to produce high-purity nickel. Ferronickel has yet to find extensive applications in industries requiring high-purity nickel. So, it is imperative to develop technologies that can upgrade ferronickel through electrolytic processes. Ferronickel, consisting of approximately 18% Ni and 80% Fe, represents an abundant but underutilized resource for high-grade nickel applications. Electrolysis was conducted using ferronickel anodes and graphite cathodes in 2 M HCl, followed by oxidation with H2O2 and precipitation with NaOH under varying pH (4.4 and 4.7) and temperature (40–70°C) conditions. Results demonstrated that the Ni concentration increased linearly from 5.36 g/L to 32.57 g/L over 8 hours of electrolysis, while Fe concentration rapidly increased and stabilized around 84.8 g/L after 3 hours. XRD analysis revealed improved crystallinity at higher temperatures, predominantly forming FeO2 and NiO phases at 70°C. XRF analysis confirmed effective iron removal, achieving 78.91% Fe precipitation at pH 4.4 and 70°C, while nickel recovery was maximized at 14.60% at pH 4.7 and 70°C, but this pH is not favorable due to the Ni loss. SEM indicated finer, more homogeneous precipitate morphology at elevated temperatures. SEM imaging revealed that at pH 4.4 the precipitate formed at 40°C had a coarse, loosely packed structure with large, irregular particles (average size ≈6.50 µm). By contrast, at 70°C the precipitate was much finer and more homogeneous, with particles ~916.8 nm. The findings highlight that electrolysis follow by optimized precipitation enables efficient separation of nickel from iron, offering a promising alternative route for upgrading ferronickel without relying on HPAL or matte processes. This approach contributes to diversifying nickel supply chains and promoting sustainable raw material utilization using local content
References
- Chung, J. (2025). The Mineral Industry of Indonesia. U.S. Geological Survey. Available at: https://pubs.usgs.gov/myb/vol3/2022/myb3-2022-indonesia.pdf
- Arif, I. (2018). Nikel Indonesia. Gramedia Pustaka Utama, 276.
- Handayani, L. (2024). Ditjen ILMATE: 44 Smelter Nikel Beroperasi di Indonesia. Media Nikel Indonesia. Available at: https://nikel.co.id/2024/03/20/ditjen-ilmate-44-smelter-nikel-beroperasi-di-indonesia/
- Xue, Y., Zhu, D., Pan, J., Guo, Z., Yang, C., Tian, H. et al. (2020). Effective Utilization of Limonitic Nickel Laterite via Pressurized Densification Process and Its Relevant Mechanism. Minerals, 10 (9), 750. https://doi.org/10.3390/min10090750
- Moussoulos, L. (1975). A process for the production of electrolytic nickel from ferronickel. Metallurgical Transactions B, 6 (4), 641–645. https://doi.org/10.1007/bf02913860
- Han, H., Sun, W., Hu, Y., Cao, X., Tang, H., Liu, R., Yue, T. (2016). Magnetite precipitation for iron removal from nickel-rich solutions in hydrometallurgy process. Hydrometallurgy, 165, 318–322. https://doi.org/10.1016/j.hydromet.2016.01.006
- Das, G. K., Li, J. (2023). Iron Removal as Goethite from Synthetic Laterite Leach Solutions. ACS Omega, 8 (13), 11931–11940. https://doi.org/10.1021/acsomega.2c07595
- Zunaidi, M. A., Setiawan, I., Oediyani, S., Irawan, J., Rhamdani, A. R., Syahid, A. N. (2022). Iron removal process from nickel pregnant leach solution using sodium hydroxide. Metalurgi, 37 (3). https://doi.org/10.14203/metalurgi.v37i3.665
- Viswanath, S. G., Jachak, M. M. (2013). Electrodeposition of nickel powder from nickel sulphate solution in presence of glycerol and sulphuric acid. Metall. Mater. Eng., 19 (3), 233–248.
- Shih, Y.-J., Chien, S.-K., Jhang, S.-R., Lin, Y.-C. (2019). Chemical leaching, precipitation and solvent extraction for sequential separation of valuable metals in cathode material of spent lithium ion batteries. Journal of the Taiwan Institute of Chemical Engineers, 100, 151–159. https://doi.org/10.1016/j.jtice.2019.04.017
- Partinen, J., Halli, P., Wilson, B. P., Lundström, M. (2023). The impact of chlorides on NMC leaching in hydrometallurgical battery recycling. Minerals Engineering, 202, 108244. https://doi.org/10.1016/j.mineng.2023.108244
- Astini, V., Meirawati, S., Nengsih, S., -, A., -, H., Soedarsono, J. W. M., Zulfia, A. (2024). Influence of Electrolyte Molarity and Applied Voltage on the Purification of Ferronickel by Electrolysis Method. Metalurgi, 39 (1), 7. https://doi.org/10.55981/metalurgi.2024.742
- Linnikov, О. D., Rodina, I. V., Zakharova, G. S., Mikhalev, K. N., Baklanova, I. V., Kuznetsova, Y. V. et al. (2022). Coagulation removal of nickel (II) ions by ferric chloride: Efficiency and mechanism. Water Environment Research, 94 (12). https://doi.org/10.1002/wer.10827
- Sanz-Medel, A., Pereiro, R. (2014). Atomic absorption spectrometry: An introduction. Momentum Press, 205.
- Luger, P. (2014). Modern X-Ray Analysis on Single Crystals. De Gruyter. https://doi.org/10.1515/9783110308280
- Speck, F. D., Dettelbach, K. E., Sherbo, R. S., Salvatore, D. A., Huang, A., Berlinguette, C. P. (2017). On the Electrolytic Stability of Iron-Nickel Oxides. Chem, 2 (4), 590–597. https://doi.org/10.1016/j.chempr.2017.03.006
- Ali, A., Zhang, N., Santos, R. M. (2023). Mineral Characterization Using Scanning Electron Microscopy (SEM): A Review of the Fundamentals, Advancements, and Research Directions. Applied Sciences, 13 (23), 12600. https://doi.org/10.3390/app132312600
- P1: Standard Reduction Potentials by Element. LibreTexts. Available at: https://chem.libretexts.org/Ancillary_Materials/Reference/Reference_Tables/Electrochemistry_Tables/P1%3A_Standard_Reduction_Potentials_by_Element
- Bösing, I. (2023). Modeling electrochemical oxide film growth—passive and transpassive behavior of iron electrodes in halide-free solution. Npj Materials Degradation, 7 (1). https://doi.org/10.1038/s41529-023-00369-y
- Shu, R., Zhang, Q., Ma, L., Xu, Y., Chen, P., Wang, C., Wang, T. (2016). Insight into the solvent, temperature and time effects on the hydrogenolysis of hydrolyzed lignin. Bioresource Technology, 221, 568–575. https://doi.org/10.1016/j.biortech.2016.09.043
- Pangaribuan, R. H., Patrick, J., Prasetyo, A. B., Maksum, A., Munir, B., Soedarsono, J. W. (2018). The effect of NaOH (natrium hydroxide) to slag nickel pyrometallurgy in different temperature and additive ratio. E3S Web of Conferences, 67, 03052. https://doi.org/10.1051/e3sconf/20186703052
- Takeno, N. (2005). Atlas of Eh-pH diagrams. Intercomparison of thermodynamic databases. Geological Survey of Japan Open File Report No. 419. National Institute of Advanced Industrial Science and Technology. Available at: https://www.nrc.gov/docs/ML1808/ML18089A638.pdf
- Vajglová, Z., Gauli, B., Mäki-Arvela, P., Kumar, N., Eränen, K., Wärnå, J. et al. (2023). Interactions between Iron and Nickel in Fe–Ni Nanoparticles on Y Zeolite for Co-Processing of Fossil Feedstock with Lignin-Derived Isoeugenol. ACS Applied Nano Materials, 6 (12), 10064–10077. https://doi.org/10.1021/acsanm.3c00620
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2025 Anne Zulfia Syahrial, Vita Astini, Johny Wahyuadi M.S

This work is licensed under a Creative Commons Attribution 4.0 International License.
The consolidation and conditions for the transfer of copyright (identification of authorship) is carried out in the License Agreement. In particular, the authors reserve the right to the authorship of their manuscript and transfer the first publication of this work to the journal under the terms of the Creative Commons CC BY license. At the same time, they have the right to conclude on their own additional agreements concerning the non-exclusive distribution of the work in the form in which it was published by this journal, but provided that the link to the first publication of the article in this journal is preserved.
A license agreement is a document in which the author warrants that he/she owns all copyright for the work (manuscript, article, etc.).
The authors, signing the License Agreement with TECHNOLOGY CENTER PC, have all rights to the further use of their work, provided that they link to our edition in which the work was published.
According to the terms of the License Agreement, the Publisher TECHNOLOGY CENTER PC does not take away your copyrights and receives permission from the authors to use and dissemination of the publication through the world's scientific resources (own electronic resources, scientometric databases, repositories, libraries, etc.).
In the absence of a signed License Agreement or in the absence of this agreement of identifiers allowing to identify the identity of the author, the editors have no right to work with the manuscript.
It is important to remember that there is another type of agreement between authors and publishers – when copyright is transferred from the authors to the publisher. In this case, the authors lose ownership of their work and may not use it in any way.








