Determination of the electrochemical dissolution feasibility of a superalloy used in turbine components in alkaline solutions with additives
DOI:
https://doi.org/10.15587/1729-4061.2025.337836Keywords:
heat-resistant superalloy, electrochemical dissolution, alkaline electrolyte, passivation, selective leaching, rheniumAbstract
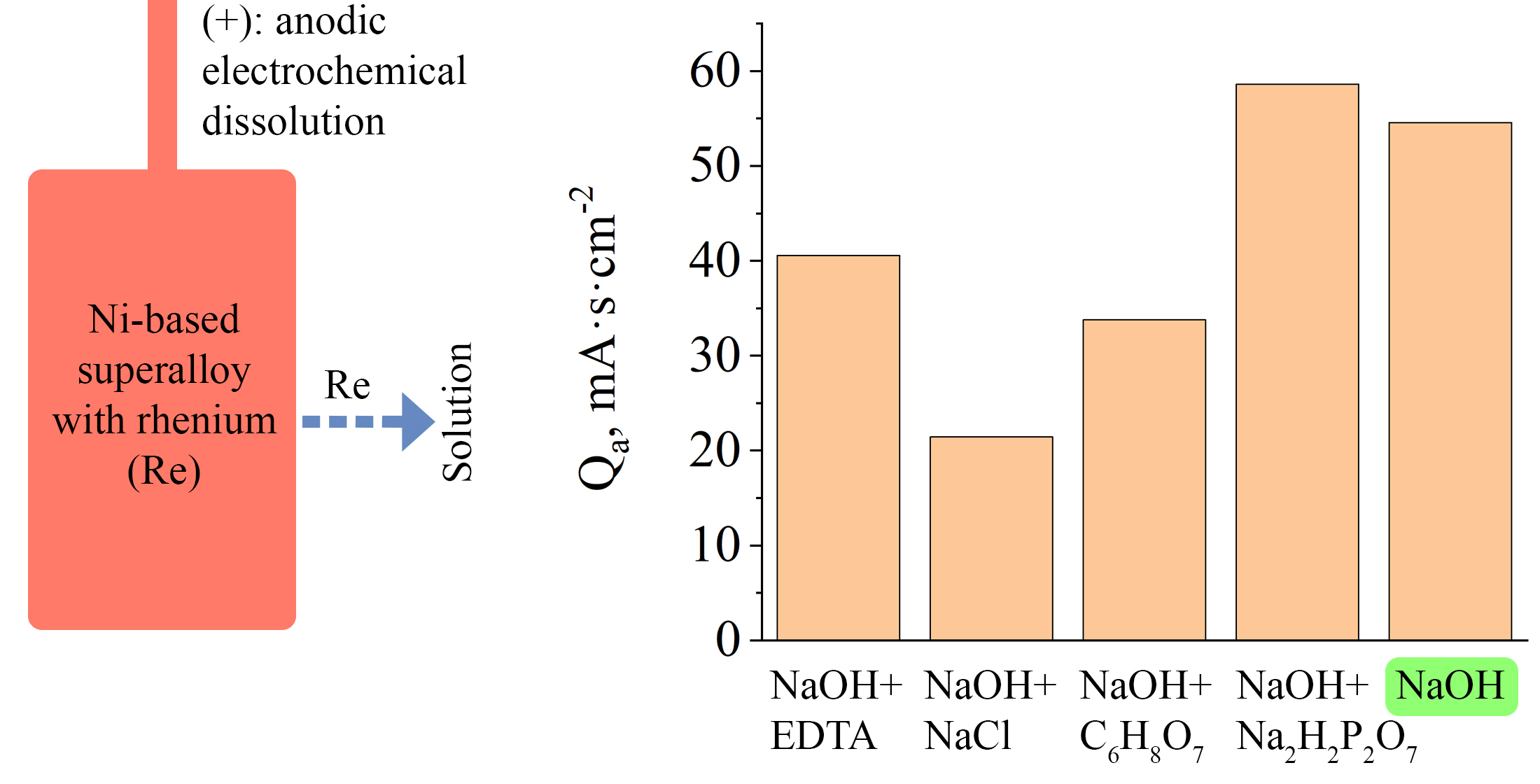
The object of this study was an electrochemical anodic dissolution of a heat-resistant nickel-based superalloy (≈62 wt.%), recovered from destroyed components of special-purpose equipment, which contains valuable metals such as Re (≈4 wt.%), Co, W, Mo, Ta, Nb, and others. The research addressed the problem of the lack of an effective electrochemical method for selectively extracting these components, particularly rhenium and other valuable elements, from such an alloy in alkaline media. The anodic behavior of the alloy was experimentally studied in 0.5 M NaOH in the presence of various complexing and activating additives (NaCl, citric acid, EDTA salt, and Na2H2P2O7). It was shown that none of the additives provided a significant acceleration of anodic dissolution. This was demonstrated by the fact that the increase in the average specific charge calculated for five cyclic voltammetry scans that contributed to alloy dissolution did not exceed 8%. In other cases, the values were significantly lower than in the base solution containing only alkali. It was established that the anodic dissolution process has a surface-selective nature: Ni, Co, Cr, Re, and Al are leached into the electrolyte, while a residual surface layer enriched in W, Ta, Nb, and Mo forms, hindering further dissolution. X-ray fluorescence analysis data confirmed changes in the chemical composition (Ni content decreased to ≈48 wt.%, W increased from ≈9 to ≈20 wt.% on the surface). Theoretical justification of the results is provided, based on the physicochemical properties of compounds that may form during anodic dissolution in the presence of additives. The absence of an activating effect from the additives suggests the need for further studies on pure NaOH. The obtained data are of practical importance for the selective separation of superalloy elements before further processing
References
- Li, J., Zhou, L., Lu, N., Song, W., Liang, J., Zhou, Y. et al. (2025). Advances and challenges in energy field assisted additive manufacturing nickel-based superalloys: Printability, microstructure, and performance. Journal of Materials Science & Technology, 239, 124–152. https://doi.org/10.1016/j.jmst.2025.03.010
- Kovalenko, V., Kotok, V. (2019). Influence of the carbonate ion on characteristics of electrochemically synthesized layered (α+β) nickel hydroxide. Eastern-European Journal of Enterprise Technologies, 1 (6 (97)), 40–46. https://doi.org/10.15587/1729-4061.2019.155738
- Torralba, J. M., Meza, A., Kumaran, S. V., Mostafaei, A., Mohammadzadeh, A. (2025). From high-entropy alloys to alloys with high entropy: A new paradigm in materials science and engineering for advancing sustainable metallurgy. Current Opinion in Solid State and Materials Science, 36, 101221. https://doi.org/10.1016/j.cossms.2025.101221
- Hu, C., Wei, Y., Cai, H., Chen, L., Wang, X., Zhang, X. et al. (2021). Research Progress of Platinum-Based Superalloys for High Temperature Applications. Johnson Matthey Technology Review, 65 (4), 535–555. https://doi.org/10.1595/205651321x16221908118376
- The Consequences of China’s new rare earths export restrictions. Center for Strategic and International Studies (CSIS). Available at: https://www.csis.org/analysis/consequences-chinas-new-rare-earths-export-restrictions
- Babiy, K. V., Bubnova, O. A., Malieiev, Ye. V., Riumina, D. M., Levchenko, K. S., Ikol, O. O. (2024). Resursy stratehichnykh korysnykh kopalyn Ukrainy. Dnipro, 324. Available at: https://www.researchgate.net/publication/390199010_Resursi_strategicnih_korisnih_kopalin_Ukraini
- Kotok, V., Kovalenko, V. (2018). A study of the effect of tungstate ions on the electrochromic properties of Ni(OH)2 films. Eastern-European Journal of Enterprise Technologies, 5 (12 (95)), 18–24. https://doi.org/10.15587/1729-4061.2018.145223
- Pure Ni Powder. Additive Manufacturing Material. Available at: https://am-material.com/316l-powder/pure-ni-powder-20240923/
- LME Nickel. London Metal Exchange. Available at: https://www.lme.com/Metals/Non-ferrous/LME-Nickel#Summary
- Kotok, V., Butyrina, T., Sknar, Y., Demchyshyna, O., Liashenko, A., Sukha, I. (2024). Determination of processing conditions for a heat-resistant superalloy used in turbine elements. Eastern-European Journal of Enterprise Technologies, 5 (12 (131)), 6–12. https://doi.org/10.15587/1729-4061.2024.313452
- Li, D., Li, G., Wei, X., Ma, B., Huang, C., Chen, W. et al. (2024). Long-term aging behavior and mechanism of CMSX-4 nickel-based single crystal superalloy at 950 ℃ and 1050 ℃. Journal of Alloys and Compounds, 1004, 175763. https://doi.org/10.1016/j.jallcom.2024.175763
- Nychka, J. A., Clarke, D. R., Meier, G. H. (2008). Spallation and transient oxide growth on PWA 1484 superalloy. Materials Science and Engineering: A, 490 (1-2), 359–368. https://doi.org/10.1016/j.msea.2008.01.043
- Stoller, V., Olbrich, A., Meese-Marktscheffel, J., Mathy, W., Erb, M., Nietfeld, G., Gille, G. (2003). Pat. No. EP1312686A2. Electrochemical dissolution process for disintegrating superalloy scraps. Available at: https://patents.google.com/patent/EP1312686A2/en
- Xu, W., Gao, K., Chen, K., Lu, J., Li, J., Li, B. et al. (2021). Study on Electrochemical Dissolution Mechanism of Nickel-Based Superalloy Scrap. SSRN Electronic Journal. https://doi.org/10.2139/ssrn.3986790
- Agapova, L. Y., Kilibayeva, S. K., Abisheva, Z. S., Sharipova, A. S. (2020). Complex electrochemical processing of technogenic wastes of rhenium-containing heat-resistant nickel alloys. Non-Ferrous Metals, 24–30. https://doi.org/10.17580/nfm.2020.01.04
- Al-Kharafi, F. M., Ateya, B. G., Allah, R. M. A. (2004). Selective dissolution of brass in salt water. Journal of Applied Electrochemistry, 34 (1), 47–53. https://doi.org/10.1023/b:jach.0000005616.41240.d0
- Zaky, A. (2013). Electrochemical Dissolution and Passivation of Cu-Ni Alloys in Sodium Sulphate Aqueous Solution. Universal Journal of Chemistry, 1 (1), 1–6. https://doi.org/10.13189/ujc.2013.010101
- Chang, J.-K., Hsu, S.-H., Sun, I.-W., Tsai, W.-T. (2008). Formation of Nanoporous Nickel by Selective Anodic Etching of the Nobler Copper Component from Electrodeposited Nickel−Copper Alloys. The Journal of Physical Chemistry C, 112 (5), 1371–1376. https://doi.org/10.1021/jp0772474
- El-Sayed, A.-R., Abd El-Lateef, H. M., Mohran, H. S. (2015). Effect of nickel content on the anodic dissolution and passivation of zinc–nickel alloys in alkaline solutions by potentiodynamic and potentiostatic techniques. Bulletin of Materials Science, 38 (2), 379–391. https://doi.org/10.1007/s12034-014-0814-7
- Yagi, R., Okabe, T. H. (2024). Review: Rhenium and its smelting and recycling technologies. International Materials Reviews, 69 (2), 142–177. https://doi.org/10.1177/09506608241229042
- Yi, A., Jiang, H. (2023). Rhenium-molybdenum separation in an alkaline leaching solution of a waste superalloy by N263 extraction. Arabian Journal of Chemistry, 16 (3), 104516. https://doi.org/10.1016/j.arabjc.2022.104516
- Maltseva, T. (2025). Proposed composition of complexes and ion associates in citric acid solutions containing nickel sulfamate and potassium perrhenate. Ukrainian Chemistry Journal, 91 (4), 61–71. https://doi.org/10.33609/2708-129x.91.4.2025.61-71
- Nahle, A. (1998). Effect of EDTA on the electrochemical dissolution of tin in NaOH solution. Bulletin of Electrochemistry, 14 (2), 52–56.
- Stankovic, S., Grgur, B., Krstajic, N., Vojnovic, M. (2003). Kinetics of the zinc anodic dissolution reaction in near neutral EDTA solutions. Journal of the Serbian Chemical Society, 68 (3), 207–218. https://doi.org/10.2298/jsc0303207s
- Rong, H., Zhang, C., Sun, Y., Wu, L., Lian, B., Wang, Y., Chen, Y. et al. (2022). Electrochemical degradation of Ni-EDTA complexes in electroless plating wastewater using PbO2-Bi electrodes. Chemical Engineering Journal, 431, 133230. https://doi.org/10.1016/j.cej.2021.133230
- Ni2P2O7 Solubility - Is Nickel(II) pyrophosphate Soluble? ChemicalAid. Available at: https://www.chemicalaid.com/tools/solubility.php?hl=en&substance=Ni2P2O7
- Mikhailov, I. F. (2016). Perspectives of development of X-ray analysis for material composition. Functional Materials, 23 (1), 5–14. https://doi.org/10.15407/fm23.01.005
- Kovalenko, V., Kotok, V., Yeroshkina, A., Zaychuk, A. (2017). Synthesis and characterisation of dyeintercalated nickelaluminium layereddouble hydroxide as a cosmetic pigment. Eastern-European Journal of Enterprise Technologies, 5 (12 (89)), 27–33. https://doi.org/10.15587/1729-4061.2017.109814
- Kotok, V., Kovalenko, V. (2018). Definition of the aging process parameters for nickel hydroxide in the alkaline medium. Eastern-European Journal of Enterprise Technologies, 2 (12 (92)), 54–60. https://doi.org/10.15587/1729-4061.2018.127764
- Kotok, V. A., Kovalenko, V. L., Solovov, V. A., Kovalenko, P. V., Ananchenko, B. A. (2018). Effect of deposition time on properties of electrochromic nickel hydroxide films prepared by cathodic template synthesis. ARPN Journal of Engineering and Applied Sciences, 13 (9), 3076–3086. Available at: https://www.arpnjournals.org/jeas/research_papers/rp_2018/jeas_0518_7034.pdf
- Kovalenko, V., Kotok, V. (2017). Selective anodic treatment of W(WC)-based superalloy scrap. Eastern-European Journal of Enterprise Technologies, 1 (5 (85)), 53–58. https://doi.org/10.15587/1729-4061.2017.91205
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2025 Valerii Kotok, Yuri Sknar, Tatyana Butyrina, Irina Sknar, Oksana Demchyshyna, Ella Chasova

This work is licensed under a Creative Commons Attribution 4.0 International License.
The consolidation and conditions for the transfer of copyright (identification of authorship) is carried out in the License Agreement. In particular, the authors reserve the right to the authorship of their manuscript and transfer the first publication of this work to the journal under the terms of the Creative Commons CC BY license. At the same time, they have the right to conclude on their own additional agreements concerning the non-exclusive distribution of the work in the form in which it was published by this journal, but provided that the link to the first publication of the article in this journal is preserved.
A license agreement is a document in which the author warrants that he/she owns all copyright for the work (manuscript, article, etc.).
The authors, signing the License Agreement with TECHNOLOGY CENTER PC, have all rights to the further use of their work, provided that they link to our edition in which the work was published.
According to the terms of the License Agreement, the Publisher TECHNOLOGY CENTER PC does not take away your copyrights and receives permission from the authors to use and dissemination of the publication through the world's scientific resources (own electronic resources, scientometric databases, repositories, libraries, etc.).
In the absence of a signed License Agreement or in the absence of this agreement of identifiers allowing to identify the identity of the author, the editors have no right to work with the manuscript.
It is important to remember that there is another type of agreement between authors and publishers – when copyright is transferred from the authors to the publisher. In this case, the authors lose ownership of their work and may not use it in any way.








