Закономірності електрохімічного синтезу тонкоплівкових фотокаталітичних матеріалів на основі гетерооксидних сполук титану
DOI:
https://doi.org/10.15587/1729-4061.2022.269942Ключові слова:
плазмо-електролітне оксидування, гетерооксиди титану, фотокаталізатор гетерооксидний, морфологія фотокаталізатора, азобарвник, допантАнотація
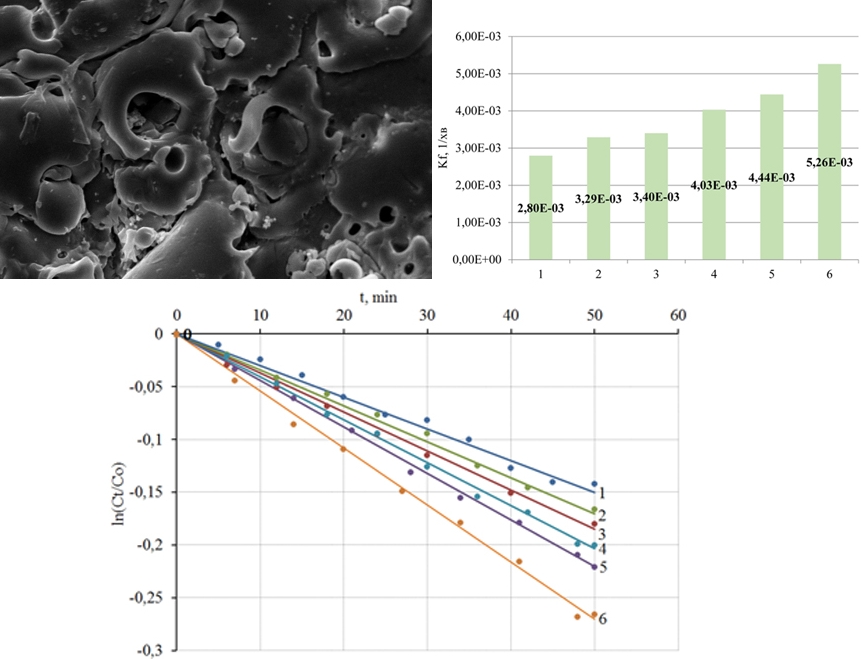
Досліджено процеси плазмо-електролітного формування гетерооксидних покриттів на сплавах титану для фотокаталітичної деструкції природних та техногенних токсикантів. Синтез покриттів проводили з водних розчинів дифосфатів у гальваностатичному режимі. Для кількісного опису фотокаталітичних реакцій розраховано константи швидкості реакції kf з лінеаризованих залежностей ln(Ct/C0), де Ct – поточна, C0 – вихідна концентрації реагенту. Морфологію поверхні покриттів досліджено методом атомно-силової мікроскопії, а результати візуалізовано шляхом реконструкції рельєфу у вигляді 2D та 3D топографічних карт. Проаналізовано морфологічні особливості покриттів з оксиду титану (IV) та гетерооксидних композитів, що містять оксиди перехідних металів. Показано, що питома площа поверхні залишається ефективним фактором регулювання фотокаталітичної активності покриттів. Встановлення морфології гетерооксидних композитів, а також методів управління цим параметром цільового продукту є незмінною складовою системного вивчення таких матеріалів при визначенні їх функціональних властивостей. Встановлено, що порівняно з оксидно-титановими покриттями, поверхневі шари яких характеризуються тороїдальною мезоструктурою, гетерооксидні композиції мають більш розвинену поверхню, що позитивно впливає на їх функціональні властивості. Наступна термообробка аналогічним чином впливає на властивості покриття. Константи швидкості фотокаталітичного розкладання азобарвника метилового жовтогарячого використано для ранжування покриттів різного складу за їх функціональними властивостями. Покриття з TiO2·ZnO виявили найвищу каталітичну активність серед досліджених матеріалів – kf дорівнює 5.26 10-3 хв-1, що в декілька разів перевершує відповідне значення для TiO2
Посилання
- Karakurkchi, A., Sakhnenko, M., Ved, M., Galak, A., Petrukhin, S. (2017). Application of oxide-metallic catalysts on valve metals for ecological catalysis. Eastern-European Journal of Enterprise Technologies, 5 (10 (89)), 12–18. doi: https://doi.org/10.15587/1729-4061.2017.109885
- Khimach, N. Yu., Polunkin, Ye. V. (2012). Nanostrukturovani katalizatory. Katalyz y neftekhymyia, 21, 86–98.
- Ved, M. V. (2017). Functional mixed cobalt and aluminum oxide coatings for environmental safety. Functional Materials, 24 (2), 005–310. doi: https://doi.org/10.15407/fm24.02.303
- Ved, M. V., Sakhnenko, M. D. (2010). Katalitychni ta zakhysni pokryttia splavamy i skladnymy oksydamy: elektrokhimichnyi syntez, prohnozuvannia vlastyvostei. Kharkiv: NTU „KhPI”, 272.
- Halak, O., Menshov, S. (2019). The use of photocatalytic technology for the disintegration of hazardous chemical substances. International Scientific Conference. doi: https://doi.org/10.30525/978-9934-588-11-2_9
- Kadhim S. H. (2016). Preparation and Characterization of Pure and Na2O Doped Co3O4 Spinel Supported Catalyst for Photocatalytic Degradation of Reactive Yellow Dye 145. International Journal of ChemTech Research, 9 (12), 754–766. Available at: https://www.sphinxsai.com/2016/ch_vol9_no12/2/(754-766)V9N12CT.pdf
- Qu, Y., Duan, X. (2013). Progress, challenge and perspective of heterogeneous photocatalysts. Chem. Soc. Rev., 42 (7), 2568–2580. doi: https://doi.org/10.1039/c2cs35355e
- Khairy, M., Zakaria, W. (2014). Effect of metal-doping of TiO2 nanoparticles on their photocatalytic activities toward removal of organic dyes. Egyptian Journal of Petroleum, 23 (4), 419–426. doi: https://doi.org/10.1016/j.ejpe.2014.09.010
- Iurascu, B., Siminiceanu, I., Vione, D., Vicente, M. A., Gil, A. (2009). Phenol degradation in water through a heterogeneous photo-Fenton process catalyzed by Fe-treated laponite. Water Research, 43 (5), 1313–1322. doi: https://doi.org/10.1016/j.watres.2008.12.032
- Grishina, E. P., Kudryakova, N. O., Rumyantsev, P. A., Zhirov, A. V., Gladiy, Yu. P., Belkin, P. N. (2013). Photoelectrochemical properties of oxide films formed by anode plasma electrolytic oxidation on titanium in water solutions. Surface Engineering and Applied Electrochemistry, 49 (1), 83–90. doi: https://doi.org/10.3103/s1068375512060026
- Marcì, G., Augugliaro, V., López-Muñoz, M. J., Martín, C., Palmisano, L., Rives, V. et al. (2001). Preparation Characterization and Photocatalytic Activity of Polycrystalline ZnO/TiO2 Systems. 2. Surface, Bulk Characterization, and 4-Nitrophenol Photodegradation in Liquid−Solid Regime. The Journal of Physical Chemistry B, 105 (5), 1033–1040. doi: https://doi.org/10.1021/jp003173j
- Meng, T., Xie, P., Qin, H., Liu, H., Hua, W., Li, X., Ma, Z. (2016). Fe2O3/SiO2 nanowires formed by hydrothermally transforming SiO2 spheres in the presence of Fe3+: Synthesis, characterization, and catalytic properties. Journal of Molecular Catalysis A: Chemical, 421, 109–116. doi: https://doi.org/10.1016/j.molcata.2016.05.017
- Kandy, M. M., Gaikar, V. G. (2018). Photocatalytic reduction of CO2 using CdS nanorods on porous anodic alumina support. Materials Research Bulletin, 102, 440–449. doi: https://doi.org/10.1016/j.materresbull.2018.02.054
- He, J., Luo, Q., Cai, Q. Z., Li, X. W., Zhang, D. Q. (2011). Microstructure and photocatalytic properties of WO3/TiO2 composite films by plasma electrolytic oxidation. Materials Chemistry and Physics, 129 (1-2), 242–248. doi: https://doi.org/10.1016/j.matchemphys.2011.04.011
- Paramasivam, I., Jha, H., Liu, N., Schmuki, P. (2012). A Review of Photocatalysis using Self-organized TiO2Nanotubes and Other Ordered Oxide Nanostructures. Small, 8 (20), 3073–3103. doi: https://doi.org/10.1002/smll.201200564
- Vasilyeva, M. S., Rudnev, V. S., Tarabrina, D. A. (2017). Photocatalytic properties of Zn- and Cd-containing oxide layers on titanium formed by plasma electrolytic oxidation. Protection of Metals and Physical Chemistry of Surfaces, 53 (4), 711–715. doi: https://doi.org/10.1134/s2070205117040232
- Zaleska, A. (2008). Doped-TiO2: A Review. Recent Patents on Engineering, 2 (3), 157–164. doi: https://doi.org/10.2174/187221208786306289
- Karakurkchi, A. V. (2015). Functional properties of multicomponent galvanic alloys of iron with molybdenum and tungsten. Functional Materials, 22 (2), 181–187. doi: https://doi.org/10.15407/fm22.02.181
- Zhang, F., Wang, X., Liu, H., Liu, C., Wan, Y., Long, Y., Cai, Z. (2019). Recent Advances and Applications of Semiconductor Photocatalytic Technology. Applied Sciences, 9 (12), 2489. doi: https://doi.org/10.3390/app9122489
- Xiao, F.-X. (2012). Construction of Highly Ordered ZnO–TiO2 Nanotube Arrays (ZnO/TNTs) Heterostructure for Photocatalytic Application. ACS Applied Materials & Interfaces, 4 (12), 7055–7063. doi: https://doi.org/10.1021/am302462d
- Fujishima, A., Zhang, X. (2006). Titanium dioxide photocatalysis: present situation and future approaches. Comptes Rendus Chimie, 9 (5-6), 750–760. doi: https://doi.org/10.1016/j.crci.2005.02.055
- Asahi, R., Morikawa, T., Ohwaki, T., Aoki, K., Taga, Y. (2001). Visible-Light Photocatalysis in Nitrogen-Doped Titanium Oxides. Science, 293 (5528), 269–271. doi: https://doi.org/10.1126/science.1061051
- Irie, H., Watanabe, Y., Hashimoto, K. (2003). Nitrogen-Concentration Dependence on Photocatalytic Activity of TiO2-xNx Powders. The Journal of Physical Chemistry B, 107 (23), 5483–5486. doi: https://doi.org/10.1021/jp030133h
- Ihara, T. (2003). Visible-light-active titanium oxide photocatalyst realized by an oxygen-deficient structure and by nitrogen doping. Applied Catalysis B: Environmental, 42 (4), 403–409. doi: https://doi.org/10.1016/s0926-3373(02)00269-2
- Kudo, A. (2003). Photocatalyst materials for water splitting. Catalysis Surveys from Asia, 7, 31–38. doi: https://doi.org/10.1023/A:1023480507710
- Prieto, J. P., Béjar, M. G. (Eds.) (2019). Photoactive inorganic nanoparticles. Surface composition and nanosystem functionality. Elsevier. doi: https://doi.org/10.1016/c2017-0-01254-5
- Ved’, M. V., Sakhnenko, M. D., Bohoyavlens’ka, O. V., Nenastina, T. O. (2008). Modeling of the surface treatment of passive metals. Materials Science, 44 (1), 79–86. doi: https://doi.org/10.1007/s11003-008-9046-6
- Sakhnenko, N. D., Ved, M. V., Karakurkchi, A. V. (2017). Nanoscale Oxide PEO Coatings Forming from Diphosphate Electrolytes. Nanophysics, Nanomaterials, Interface Studies, and Applications, 507–531. doi: https://doi.org/10.1007/978-3-319-56422-7_38
- Yan, G., Zhang, M., Hou, J., Yang, J. (2011). Photoelectrochemical and photocatalytic properties of N+S co-doped TiO2 nanotube array films under visible light irradiation. Materials Chemistry and Physics, 129 (1-2), 553–557. doi: https://doi.org/10.1016/j.matchemphys.2011.04.063
- Yar-Mukhamedova, G. Sh., Sakhnenko, N. D., Ved, M. V. (2020). Nanocomposite electrolytic coatings with defined functional properties. Almaty: Kazakh University, 180.
- Lim, S. Y., Law, C. S., Liu, L., Markovic, M., Hedrich, C., Blick, R. H. et al. (2019). Electrochemical Engineering of Nanoporous Materials for Photocatalysis: Fundamentals, Advances, and Perspectives. Catalysts, 9 (12), 988. doi: https://doi.org/10.3390/catal9120988
- Luo, Q., Cai, Q., Li, X., Chen, X. (2014). Characterization and photocatalytic activity of large-area single crystalline anatase TiO2 nanotube films hydrothermal synthesized on Plasma electrolytic oxidation seed layers. Journal of Alloys and Compounds, 597, 101–109. doi: https://doi.org/10.1016/j.jallcom.2014.01.216
- Herrmann, J.-M. (2005). Heterogeneous photocatalysis: state of the art and present applications In honor of Pr. R.L. Burwell Jr. (1912–2003), Former Head of Ipatieff Laboratories, Northwestern University, Evanston (Ill). Topics in Catalysis, 34 (1-4), 49–65. doi: https://doi.org/10.1007/s11244-005-3788-2
- Vinu, R., Madras, G. (2010). Environmental remediation by photocatalysis. Journal of the Indian Institute of Science, 90 (2), 189–230. Available at: http://journal.iisc.ernet.in/index.php/iisc/article/view/95/92
##submission.downloads##
Опубліковано
Як цитувати
Номер
Розділ
Ліцензія
Авторське право (c) 2022 Mykola Sakhnenko, Iryna Stepanova, Alla Korogodskaya, Ann Karakurkchi, Olena Skrypnyk, Anatoly Dzheniuk, Oleksandr Halak

Ця робота ліцензується відповідно до Creative Commons Attribution 4.0 International License.
Закріплення та умови передачі авторських прав (ідентифікація авторства) здійснюється у Ліцензійному договорі. Зокрема, автори залишають за собою право на авторство свого рукопису та передають журналу право першої публікації цієї роботи на умовах ліцензії Creative Commons CC BY. При цьому вони мають право укладати самостійно додаткові угоди, що стосуються неексклюзивного поширення роботи у тому вигляді, в якому вона була опублікована цим журналом, але за умови збереження посилання на першу публікацію статті в цьому журналі.
Ліцензійний договір – це документ, в якому автор гарантує, що володіє усіма авторськими правами на твір (рукопис, статтю, тощо).
Автори, підписуючи Ліцензійний договір з ПП «ТЕХНОЛОГІЧНИЙ ЦЕНТР», мають усі права на подальше використання свого твору за умови посилання на наше видання, в якому твір опублікований. Відповідно до умов Ліцензійного договору, Видавець ПП «ТЕХНОЛОГІЧНИЙ ЦЕНТР» не забирає ваші авторські права та отримує від авторів дозвіл на використання та розповсюдження публікації через світові наукові ресурси (власні електронні ресурси, наукометричні бази даних, репозитарії, бібліотеки тощо).
За відсутності підписаного Ліцензійного договору або за відсутністю вказаних в цьому договорі ідентифікаторів, що дають змогу ідентифікувати особу автора, редакція не має права працювати з рукописом.
Важливо пам’ятати, що існує і інший тип угоди між авторами та видавцями – коли авторські права передаються від авторів до видавця. В такому разі автори втрачають права власності на свій твір та не можуть його використовувати в будь-який спосіб.









