Using the gold bullion slag from Indonesia as lithium resources with HCL leaching method
DOI:
https://doi.org/10.15587/1729-4061.2023.273491Keywords:
lithium extraction, gold bullion slag, acid leaching, secondary resources, ANOVAAbstract
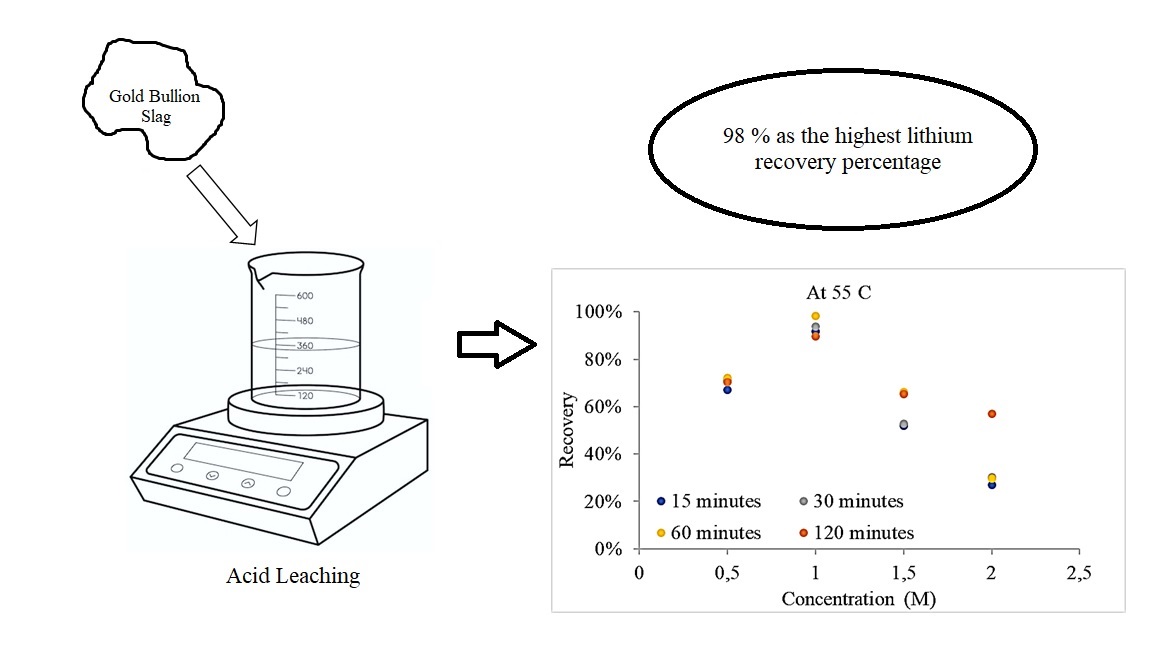
Primary resources are typically used in lithium extraction. Unfortunately, it impacts the dependency on the availability of primary resources to fulfill the lithium demand. Therefore, the use of secondary resources can be an alternative to using lithium resources. Gold bullion slag is an example of a potential secondary resource used as a lithium source because it contains 0.009 % lithium. This research aims at increasing lithium recovery from the gold bullion slag by studying the effects of various variables to enhance lithium recovery. Lithium extraction was carried out via HCl leaching process with concentrations of 0.5, 1.0, 1.5, and 2.0 M at 25, 40, 55, and 70 °C for 15, 30, 60, and 120 minutes. Inductively coupled plasma-optical emission spectrometry (ICP-OES) was used to examine lithium level, whereas scanning electron microscope equipped with energy dispersive X-ray spectroscopy (SEM-EDX) was used to look over the morphology. The significance of the recovery value was analyzed statistically using analysis of variance (ANOVA). The optimum variables to reach 98 % as the highest lithium recovery percentage are 1 M HCl at 55 °C for 60 minutes. ANOVA results on the acid concentration significance of the recovery value show that the p-value (0.001) is smaller than the alpha value (0.005). While, ANOVA results on the temperature and time significance of the recovery value show that the p-value (0.894) is greater than the alpha value (0.005) and p-value (0.9986) is greater than the alpha value (0.005), respectively. Analysis showed that variation in HCl concentration affected the lithium recovery value; however, temperature and time of leaching had an insignificant effect on lithium recovery. These data show that slag can be used as alternative resources to produce the lithium
Supporting Agency
- The authors express their gratitude for the financial support of the Directorate of Research and Development Universitas Indonesia through PUTI (Publikasi Terindeks Internasional) Pascasarjana in 2022; the contract number is NKB-319/UN2.RST/HKP.05.00/2022.
References
- Indonesia Sets Ambitious Plan to Increase Electric Vehicle Sales By 2030. Tempo.co. Available at: https://en.tempo.co/read/1607001/indonesia-sets-ambitious-plan-to-increase-electric-vehicle-sales-by-2030
- Hocking, M., Kan, J., Young, P., Terry, C., Begleiter, D. (2016). Lithium 101. Deutsche Bank Market Research. Available at: http://panopus.net/assets/files/160509-Deutsches-Bank---Welcome-to-the-Lithium-Ion-Age.pdf
- Swain, B. (2017). Recovery and recycling of lithium: A review. Separation and Purification Technology, 172, 388–403. doi: https://doi.org/10.1016/j.seppur.2016.08.031
- Larcher, D., Tarascon, J.-M. (2014). Towards greener and more sustainable batteries for electrical energy storage. Nature Chemistry, 7 (1), 19–29. doi: https://doi.org/10.1038/nchem.2085
- Gao, Z., Huang, M., Yang, L., Feng, Y., Ding, Y., Shao, P., Luo, X. (2023). Review of preferentially selective lithium extraction from spent lithium batteries: Principle and performance. Journal of Energy Chemistry, 78, 253–261. doi: https://doi.org/10.1016/j.jechem.2022.11.061
- Perkins, D. N., Brune Drisse, M.-N., Nxele, T., Sly, P. D. (2014). E-Waste: A Global Hazard. Annals of Global Health, 80 (4), 286. doi: https://doi.org/10.1016/j.aogh.2014.10.001
- Kim, Y., Kim, M., Sohn, J., Park, H. (2018). Applicability of gold tailings, waste limestone, red mud, and ferronickel slag for producing glass fibers. Journal of Cleaner Production, 203, 957–965. doi: https://doi.org/10.1016/j.jclepro.2018.08.230
- Wellmer, F.-W., Hagelüken, C. (2015). The Feedback Control Cycle of Mineral Supply, Increase of Raw Material Efficiency, and Sustainable Development. Minerals, 5 (4), 815–836. doi: https://doi.org/10.3390/min5040527
- Aworn, A., Thiravetyan, P., Nakbanpote, W. (2005). Recovery of gold from gold slag by wood shaving fly ash. Journal of Colloid and Interface Science, 287 (2), 394–400. doi: https://doi.org/10.1016/j.jcis.2005.02.048
- Purnama, Y. (2016). Ekonomi hijau melalui teknologi solidifikasi tailing untuk mendukung infrastruktur hijau. ANTAM.
- Peng, C., Liu, F., Wang, Z., Wilson, B. P., Lundström, M. (2019). Selective extraction of lithium (Li) and preparation of battery grade lithium carbonate (Li2CO3) from spent Li-ion batteries in nitrate system. Journal of Power Sources, 415, 179–188. doi: https://doi.org/10.1016/j.jpowsour.2019.01.072
- Xu, X., Mu, W., Xiao, T., Li, L., Xin, H., Lei, X., Luo, S. (2022). A clean and efficient process for simultaneous extraction of Li, Co, Ni and Mn from spent Lithium-ion batteries by low-temperature NH4Cl roasting and water leaching. Waste Management, 153, 61–71. doi: https://doi.org/10.1016/j.wasman.2022.08.022
- Qu, G., Wei, Y., Liu, C., Yao, S., Zhou, S., Li, B. (2022). Efficient separation and recovery of lithium through volatilization in the recycling process of spent lithium-ion batteries. Waste Management, 150, 66–74. doi: https://doi.org/10.1016/j.wasman.2022.06.039
- Shuva, M., Asw, K. (2013). Hydrometallurgical Recovery of Value Metals from Spent Lithium Ion Batteries. American Journal of Materials Engineering and Technology, 1 (1), 8–12. Available at: https://www.researchgate.net/publication/260287016_Hydrometallurgical_Recovery_of_Value_Metals_from_Spent_Lithium_Ion_Batteries
- Chen, X., Chen, Y., Zhou, T., Liu, D., Hu, H., Fan, S. (2015). Hydrometallurgical recovery of metal values from sulfuric acid leaching liquor of spent lithium-ion batteries. Waste Management, 38, 349–356. doi: https://doi.org/10.1016/j.wasman.2014.12.023
- Shuya, L., Yang, C., Xuefeng, C., Wei, S., Yaqing, W., Yue, Y. (2020). Separation of lithium and transition metals from leachate of spent lithium-ion batteries by solvent extraction method with Versatic 10. Separation and Purification Technology, 250, 117258. doi: https://doi.org/10.1016/j.seppur.2020.117258
- Zhao, J., Qu, X., Qu, J., Zhang, B., Ning, Z., Xie, H. et al. (2019). Extraction of Co and Li2CO3 from cathode materials of spent lithium-ion batteries through a combined acid-leaching and electro-deoxidation approach. Journal of Hazardous Materials, 379, 120817. doi: https://doi.org/10.1016/j.jhazmat.2019.120817
- Natasha, N. C., Lalasari, L. H., Rohmah, M., Sudarsono, J. W. (2018). Ekstraksi Litium dari β – Spodumen Hasil Dekomposisi Batuan Sekismika Indonesia Menggunakan Aditif Natrium Sulfat [Lithium Extraction from β-Spodumene the Decomposition Product of Schist Mica Indonesia Using Sodium Sulphate as Additive]. Metalurgi, 33 (2), 69. doi: https://doi.org/10.14203/metalurgi.v33i2.429
- Natasha, N. C., Lalasari, L. H., Andriyah, L., Arini, T., Yunita, F., Haryono, D., Rinanda, F. (2021). The use of mica schist from Indonesia as raw material for lithium extraction process using sulfate roasting and acid leaching. Eastern-European Journal of Enterprise Technologies, 3 (6 (111)), 80–88. doi: https://doi.org/10.15587/1729-4061.2021.231071
- Gutt, B., Kehl, K., Ren, Q., Boesel, L. F. (2016). Using ANOVA Models To Compare and Optimize Extraction Protocols of P3HBHV from Cupriavidus necator. Industrial & Engineering Chemistry Research, 55 (39), 10355–10365. doi: https://doi.org/10.1021/acs.iecr.6b02694
- Lalasari, L. H., Rohmah, M., Setiawan, I., Natasha, N. C., Andriyah, L., Arini, T. et al. (2019). Effect of Leaching Temperature on Lithium Recovery fromLi-Montmorillonite (Bledug Kuwu’s Mud). IOP Conference Series: Materials Science and Engineering, 478, 012024. doi: https://doi.org/10.1088/1757-899x/478/1/012024
- Guo, Y., Li, F., Zhu, H., Li, G., Huang, J., He, W. (2016). Leaching lithium from the anode electrode materials of spent lithium-ion batteries by hydrochloric acid (HCl). Waste Management, 51, 227–233. doi: https://doi.org/10.1016/j.wasman.2015.11.036
- Li, L., Ge, J., Chen, R., Wu, F., Chen, S., Zhang, X. (2010). Environmental friendly leaching reagent for cobalt and lithium recovery from spent lithium-ion batteries. Waste Management, 30 (12), 2615–2621. doi: https://doi.org/10.1016/j.wasman.2010.08.008
- Ikhsan, J., Wells, J. D., Johnson, B. B., Angove, M. J. (2005). Sorption of 3-amino-1,2,4-triazole and Zn(II) onto montmorillonite. Clays and Clay Minerals, 53 (2), 137–146. doi: https://doi.org/10.1346/ccmn.2005.0530203
- Rosales, G. D., Ruiz, M. del C., Rodriguez, M. H. (2014). Novel process for the extraction of lithium from β-spodumene by leaching with HF. Hydrometallurgy, 147-148, 1–6. doi: https://doi.org/10.1016/j.hydromet.2014.04.009
- Gournis, D., Lappas, A., Karakassides, M. A., Többens, D., Moukarika, A. (2007). A neutron diffraction study of alkali cation migration in montmorillonites. Physics and Chemistry of Minerals, 35 (1), 49–58. doi: https://doi.org/10.1007/s00269-007-0197-z
- Al-Ani, T., Sarapää, O. (2008). Clay and clay mineralogy. Geologian Tutkuskeskus M19/3232/2008/41. Available at: https://www.researchgate.net/publication/292706105_Clay_and_clay_mineralogy
- Zhang, B., Xu, Y., Makuza, B., Zhu, F., Wang, H., Hong, N. et al. (2023). Selective lithium extraction and regeneration of LiCoO2 cathode materials from the spent lithium-ion battery. Chemical Engineering Journal, 452, 139258. doi: https://doi.org/10.1016/j.cej.2022.139258
- An, J. W., Kang, D. J., Tran, K. T., Kim, M. J., Lim, T., Tran, T. (2012). Recovery of lithium from Uyuni salar brine. Hydrometallurgy, 117-118, 64–70. doi: https://doi.org/10.1016/j.hydromet.2012.02.008
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2023 Nadia Natasha, Ghina Rabbani, Nofrijon Sofyan, Johny Soedarsono, Agus Prasetyo, Ahmad Maksum, Rini Riastuti, Isnanda Nuriskasari

This work is licensed under a Creative Commons Attribution 4.0 International License.
The consolidation and conditions for the transfer of copyright (identification of authorship) is carried out in the License Agreement. In particular, the authors reserve the right to the authorship of their manuscript and transfer the first publication of this work to the journal under the terms of the Creative Commons CC BY license. At the same time, they have the right to conclude on their own additional agreements concerning the non-exclusive distribution of the work in the form in which it was published by this journal, but provided that the link to the first publication of the article in this journal is preserved.
A license agreement is a document in which the author warrants that he/she owns all copyright for the work (manuscript, article, etc.).
The authors, signing the License Agreement with TECHNOLOGY CENTER PC, have all rights to the further use of their work, provided that they link to our edition in which the work was published.
According to the terms of the License Agreement, the Publisher TECHNOLOGY CENTER PC does not take away your copyrights and receives permission from the authors to use and dissemination of the publication through the world's scientific resources (own electronic resources, scientometric databases, repositories, libraries, etc.).
In the absence of a signed License Agreement or in the absence of this agreement of identifiers allowing to identify the identity of the author, the editors have no right to work with the manuscript.
It is important to remember that there is another type of agreement between authors and publishers – when copyright is transferred from the authors to the publisher. In this case, the authors lose ownership of their work and may not use it in any way.









