Використання золотого шлаку з індонезії як джерела літію з методом вилучування HCL
DOI:
https://doi.org/10.15587/1729-4061.2023.273491Ключові слова:
екстракція літію, золотий шлак, кислотне вилуговування, вторинні ресурси, ANOVAАнотація
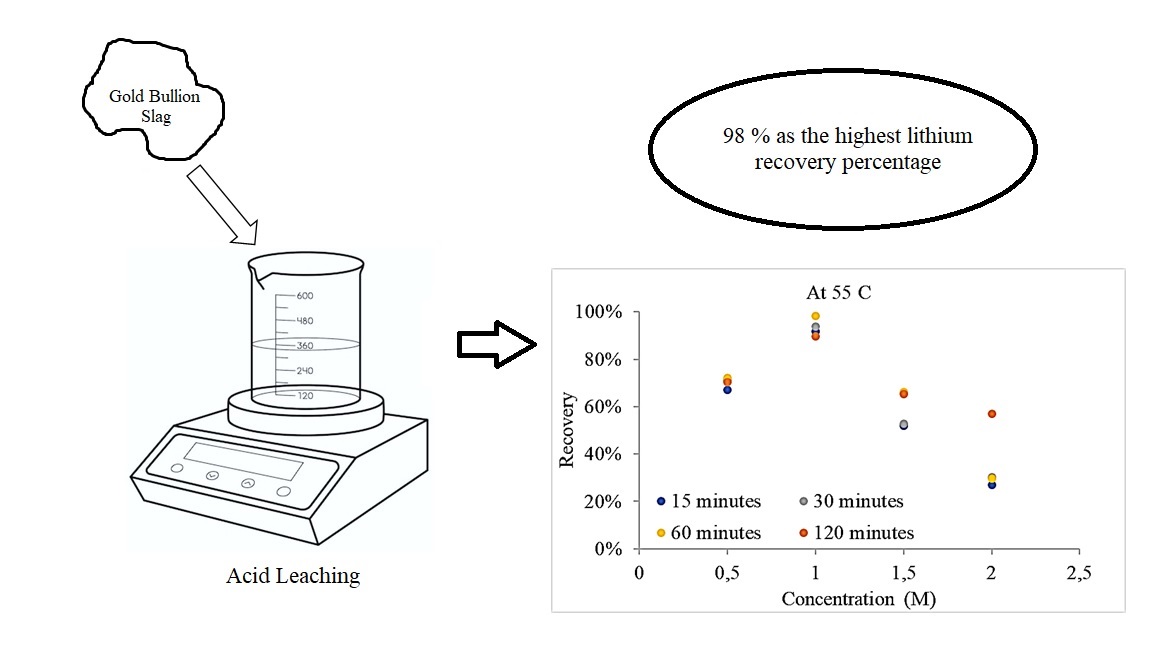
Основні ресурси зазвичай використовуються для видобутку літію. На жаль, це впливає на залежність від наявності первинних ресурсів для задоволення попиту на літій. Тому використання вторинних ресурсів може бути альтернативою використанню ресурсів літію. Золотий шлак є прикладом потенційного вторинного ресурсу, який використовується як джерело літію, оскільки він містить 0,009 % літію. Це дослідження спрямоване на збільшення вилучення літію зі шлаку золотих злитків шляхом вивчення впливу різних змінних на покращення вилучення літію. Екстракцію літію проводили за допомогою процесу вилуговування HCl з концентраціями 0,5, 1,0, 1,5 і 2,0 М при 25, 40, 55 і 70 °C протягом 15, 30, 60 і 120 хвилин. Оптично-емісійна спектрометрія з індуктивно пов’язаною плазмою використовувалася для дослідження рівня літію, тоді як скануючий електронний мікроскоп, оснащений енергодисперсійною рентгенівською спектроскопією, використовувався для перегляду морфології. Значимість значення відновлення аналізували статистично за допомогою дисперсійного аналізу (ANOVA). Оптимальними змінними для досягнення 98 %, оскільки найвищий відсоток відновлення літію є 1 М HCl при 55 °C протягом 60 хвилин. Результати ANOVA щодо значущості концентрації кислоти для значення вилучення показують, що p-значення (0,001) менше, ніж значення альфа (0,005). У той час як результати ANOVA щодо значення температури та часу значення відновлення показують, що значення p (0,894) більше, ніж значення альфа (0,005), а значення p (0,9986) більше, ніж значення альфа (0,005), відповідно . Аналіз показав, що зміна концентрації HCl вплинула на значення відновлення літію; однак температура і час вилуговування мали незначний вплив на відновлення літію. Ці дані показують, що шлак можна використовувати як альтернативний ресурс для виробництва літію
Спонсор дослідження
- The authors express their gratitude for the financial support of the Directorate of Research and Development Universitas Indonesia through PUTI (Publikasi Terindeks Internasional) Pascasarjana in 2022; the contract number is NKB-319/UN2.RST/HKP.05.00/2022.
Посилання
- Indonesia Sets Ambitious Plan to Increase Electric Vehicle Sales By 2030. Tempo.co. Available at: https://en.tempo.co/read/1607001/indonesia-sets-ambitious-plan-to-increase-electric-vehicle-sales-by-2030
- Hocking, M., Kan, J., Young, P., Terry, C., Begleiter, D. (2016). Lithium 101. Deutsche Bank Market Research. Available at: http://panopus.net/assets/files/160509-Deutsches-Bank---Welcome-to-the-Lithium-Ion-Age.pdf
- Swain, B. (2017). Recovery and recycling of lithium: A review. Separation and Purification Technology, 172, 388–403. doi: https://doi.org/10.1016/j.seppur.2016.08.031
- Larcher, D., Tarascon, J.-M. (2014). Towards greener and more sustainable batteries for electrical energy storage. Nature Chemistry, 7 (1), 19–29. doi: https://doi.org/10.1038/nchem.2085
- Gao, Z., Huang, M., Yang, L., Feng, Y., Ding, Y., Shao, P., Luo, X. (2023). Review of preferentially selective lithium extraction from spent lithium batteries: Principle and performance. Journal of Energy Chemistry, 78, 253–261. doi: https://doi.org/10.1016/j.jechem.2022.11.061
- Perkins, D. N., Brune Drisse, M.-N., Nxele, T., Sly, P. D. (2014). E-Waste: A Global Hazard. Annals of Global Health, 80 (4), 286. doi: https://doi.org/10.1016/j.aogh.2014.10.001
- Kim, Y., Kim, M., Sohn, J., Park, H. (2018). Applicability of gold tailings, waste limestone, red mud, and ferronickel slag for producing glass fibers. Journal of Cleaner Production, 203, 957–965. doi: https://doi.org/10.1016/j.jclepro.2018.08.230
- Wellmer, F.-W., Hagelüken, C. (2015). The Feedback Control Cycle of Mineral Supply, Increase of Raw Material Efficiency, and Sustainable Development. Minerals, 5 (4), 815–836. doi: https://doi.org/10.3390/min5040527
- Aworn, A., Thiravetyan, P., Nakbanpote, W. (2005). Recovery of gold from gold slag by wood shaving fly ash. Journal of Colloid and Interface Science, 287 (2), 394–400. doi: https://doi.org/10.1016/j.jcis.2005.02.048
- Purnama, Y. (2016). Ekonomi hijau melalui teknologi solidifikasi tailing untuk mendukung infrastruktur hijau. ANTAM.
- Peng, C., Liu, F., Wang, Z., Wilson, B. P., Lundström, M. (2019). Selective extraction of lithium (Li) and preparation of battery grade lithium carbonate (Li2CO3) from spent Li-ion batteries in nitrate system. Journal of Power Sources, 415, 179–188. doi: https://doi.org/10.1016/j.jpowsour.2019.01.072
- Xu, X., Mu, W., Xiao, T., Li, L., Xin, H., Lei, X., Luo, S. (2022). A clean and efficient process for simultaneous extraction of Li, Co, Ni and Mn from spent Lithium-ion batteries by low-temperature NH4Cl roasting and water leaching. Waste Management, 153, 61–71. doi: https://doi.org/10.1016/j.wasman.2022.08.022
- Qu, G., Wei, Y., Liu, C., Yao, S., Zhou, S., Li, B. (2022). Efficient separation and recovery of lithium through volatilization in the recycling process of spent lithium-ion batteries. Waste Management, 150, 66–74. doi: https://doi.org/10.1016/j.wasman.2022.06.039
- Shuva, M., Asw, K. (2013). Hydrometallurgical Recovery of Value Metals from Spent Lithium Ion Batteries. American Journal of Materials Engineering and Technology, 1 (1), 8–12. Available at: https://www.researchgate.net/publication/260287016_Hydrometallurgical_Recovery_of_Value_Metals_from_Spent_Lithium_Ion_Batteries
- Chen, X., Chen, Y., Zhou, T., Liu, D., Hu, H., Fan, S. (2015). Hydrometallurgical recovery of metal values from sulfuric acid leaching liquor of spent lithium-ion batteries. Waste Management, 38, 349–356. doi: https://doi.org/10.1016/j.wasman.2014.12.023
- Shuya, L., Yang, C., Xuefeng, C., Wei, S., Yaqing, W., Yue, Y. (2020). Separation of lithium and transition metals from leachate of spent lithium-ion batteries by solvent extraction method with Versatic 10. Separation and Purification Technology, 250, 117258. doi: https://doi.org/10.1016/j.seppur.2020.117258
- Zhao, J., Qu, X., Qu, J., Zhang, B., Ning, Z., Xie, H. et al. (2019). Extraction of Co and Li2CO3 from cathode materials of spent lithium-ion batteries through a combined acid-leaching and electro-deoxidation approach. Journal of Hazardous Materials, 379, 120817. doi: https://doi.org/10.1016/j.jhazmat.2019.120817
- Natasha, N. C., Lalasari, L. H., Rohmah, M., Sudarsono, J. W. (2018). Ekstraksi Litium dari β – Spodumen Hasil Dekomposisi Batuan Sekismika Indonesia Menggunakan Aditif Natrium Sulfat [Lithium Extraction from β-Spodumene the Decomposition Product of Schist Mica Indonesia Using Sodium Sulphate as Additive]. Metalurgi, 33 (2), 69. doi: https://doi.org/10.14203/metalurgi.v33i2.429
- Natasha, N. C., Lalasari, L. H., Andriyah, L., Arini, T., Yunita, F., Haryono, D., Rinanda, F. (2021). The use of mica schist from Indonesia as raw material for lithium extraction process using sulfate roasting and acid leaching. Eastern-European Journal of Enterprise Technologies, 3 (6 (111)), 80–88. doi: https://doi.org/10.15587/1729-4061.2021.231071
- Gutt, B., Kehl, K., Ren, Q., Boesel, L. F. (2016). Using ANOVA Models To Compare and Optimize Extraction Protocols of P3HBHV from Cupriavidus necator. Industrial & Engineering Chemistry Research, 55 (39), 10355–10365. doi: https://doi.org/10.1021/acs.iecr.6b02694
- Lalasari, L. H., Rohmah, M., Setiawan, I., Natasha, N. C., Andriyah, L., Arini, T. et al. (2019). Effect of Leaching Temperature on Lithium Recovery fromLi-Montmorillonite (Bledug Kuwu’s Mud). IOP Conference Series: Materials Science and Engineering, 478, 012024. doi: https://doi.org/10.1088/1757-899x/478/1/012024
- Guo, Y., Li, F., Zhu, H., Li, G., Huang, J., He, W. (2016). Leaching lithium from the anode electrode materials of spent lithium-ion batteries by hydrochloric acid (HCl). Waste Management, 51, 227–233. doi: https://doi.org/10.1016/j.wasman.2015.11.036
- Li, L., Ge, J., Chen, R., Wu, F., Chen, S., Zhang, X. (2010). Environmental friendly leaching reagent for cobalt and lithium recovery from spent lithium-ion batteries. Waste Management, 30 (12), 2615–2621. doi: https://doi.org/10.1016/j.wasman.2010.08.008
- Ikhsan, J., Wells, J. D., Johnson, B. B., Angove, M. J. (2005). Sorption of 3-amino-1,2,4-triazole and Zn(II) onto montmorillonite. Clays and Clay Minerals, 53 (2), 137–146. doi: https://doi.org/10.1346/ccmn.2005.0530203
- Rosales, G. D., Ruiz, M. del C., Rodriguez, M. H. (2014). Novel process for the extraction of lithium from β-spodumene by leaching with HF. Hydrometallurgy, 147-148, 1–6. doi: https://doi.org/10.1016/j.hydromet.2014.04.009
- Gournis, D., Lappas, A., Karakassides, M. A., Többens, D., Moukarika, A. (2007). A neutron diffraction study of alkali cation migration in montmorillonites. Physics and Chemistry of Minerals, 35 (1), 49–58. doi: https://doi.org/10.1007/s00269-007-0197-z
- Al-Ani, T., Sarapää, O. (2008). Clay and clay mineralogy. Geologian Tutkuskeskus M19/3232/2008/41. Available at: https://www.researchgate.net/publication/292706105_Clay_and_clay_mineralogy
- Zhang, B., Xu, Y., Makuza, B., Zhu, F., Wang, H., Hong, N. et al. (2023). Selective lithium extraction and regeneration of LiCoO2 cathode materials from the spent lithium-ion battery. Chemical Engineering Journal, 452, 139258. doi: https://doi.org/10.1016/j.cej.2022.139258
- An, J. W., Kang, D. J., Tran, K. T., Kim, M. J., Lim, T., Tran, T. (2012). Recovery of lithium from Uyuni salar brine. Hydrometallurgy, 117-118, 64–70. doi: https://doi.org/10.1016/j.hydromet.2012.02.008
##submission.downloads##
Опубліковано
Як цитувати
Номер
Розділ
Ліцензія
Авторське право (c) 2023 Nadia Natasha, Ghina Rabbani, Nofrijon Sofyan, Johny Soedarsono, Agus Prasetyo, Ahmad Maksum, Rini Riastuti, Isnanda Nuriskasari

Ця робота ліцензується відповідно до Creative Commons Attribution 4.0 International License.
Закріплення та умови передачі авторських прав (ідентифікація авторства) здійснюється у Ліцензійному договорі. Зокрема, автори залишають за собою право на авторство свого рукопису та передають журналу право першої публікації цієї роботи на умовах ліцензії Creative Commons CC BY. При цьому вони мають право укладати самостійно додаткові угоди, що стосуються неексклюзивного поширення роботи у тому вигляді, в якому вона була опублікована цим журналом, але за умови збереження посилання на першу публікацію статті в цьому журналі.
Ліцензійний договір – це документ, в якому автор гарантує, що володіє усіма авторськими правами на твір (рукопис, статтю, тощо).
Автори, підписуючи Ліцензійний договір з ПП «ТЕХНОЛОГІЧНИЙ ЦЕНТР», мають усі права на подальше використання свого твору за умови посилання на наше видання, в якому твір опублікований. Відповідно до умов Ліцензійного договору, Видавець ПП «ТЕХНОЛОГІЧНИЙ ЦЕНТР» не забирає ваші авторські права та отримує від авторів дозвіл на використання та розповсюдження публікації через світові наукові ресурси (власні електронні ресурси, наукометричні бази даних, репозитарії, бібліотеки тощо).
За відсутності підписаного Ліцензійного договору або за відсутністю вказаних в цьому договорі ідентифікаторів, що дають змогу ідентифікувати особу автора, редакція не має права працювати з рукописом.
Важливо пам’ятати, що існує і інший тип угоди між авторами та видавцями – коли авторські права передаються від авторів до видавця. В такому разі автори втрачають права власності на свій твір та не можуть його використовувати в будь-який спосіб.









