Development of Annona muricata Linn as green corrosion inhibitor under produced water: inhibition performance and adsorption model
DOI:
https://doi.org/10.15587/1729-4061.2023.278911Keywords:
green corrosion inhibitors, organic corrosion inhibitors, Annona muricata Linn, soursop adsorption inhibitionAbstract
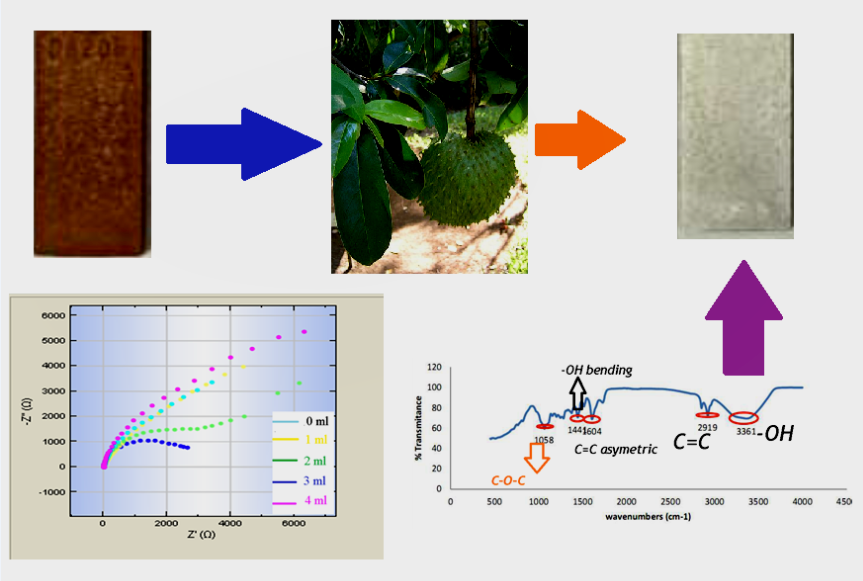
This work used soursop as a green corrosion inhibitor to protect API 5L Grade A from detrimental corrodent under produced water. Despite the effectiveness of inorganic inhibitors, recent evidence on their toxicity test suggests that implementing organic inhibitors is substantial to replace synthetic corrosion inhibitors. However, soursop utilization as a green corrosion inhibitor is poorly understood due to the lack of a comprehensive extraction mode and inhibitive mechanism. Several tests were conducted, including weight loss, potentiodynamic polarization, and electrochemical impedance spectroscopy (EIS), to unveil the nature of corrosion inhibition. Fourier Transform Infra-Red Spectroscopy revealed the dominant functional groups to bind with the substrate. The potentiodynamic polarization results show that the inhibitor is a mixed-type inhibitor that influences the anodic and cathodic reactions. The weight loss test showcases the highest inhibition efficiency of 52.62 % upon adding 2 ml inhibitors upon eight observation days. The polarization and EIS results provide that the inhibitor reduces the corrosion rate with higher inhibition of 88.52 %. The mentioned result is associated with the attachment of non-polar and polar Annona muricata Linn functional groups. The primary functional group involves C=O, C‒C and –O.H., which actively bonded to the metal's surface. The aromatic group at a wavenumber of 1,050 and 1,090 cm-1 shows ether's presence and behaves as an adsorption center. In this work, combining three solvents, hexane, acetone, and ethanol, elicits the complete extraction of the predominant compound from soursop
References
- Liu, H., Gu, T., Zhang, G., Wang, W., Dong, S., Cheng, Y., Liu, H. (2016). Corrosion inhibition of carbon steel in CO2-containing oilfield produced water in the presence of iron-oxidizing bacteria and inhibitors. Corrosion Science, 105, 149–160. doi: https://doi.org/10.1016/j.corsci.2016.01.012
- Verma, C., Ebenso, E. E., Bahadur, I., Quraishi, M. A. (2018). An overview on plant extracts as environmental sustainable and green corrosion inhibitors for metals and alloys in aggressive corrosive media. Journal of Molecular Liquids, 266, 577–590. doi: https://doi.org/10.1016/j.molliq.2018.06.110
- Fakhru’l-Razi, A., Pendashteh, A., Abdullah, L. C., Biak, D. R. A., Madaeni, S. S., Abidin, Z. Z. (2009). Review of technologies for oil and gas produced water treatment. Journal of Hazardous Materials, 170 (2-3), 530–551. doi: https://doi.org/10.1016/j.jhazmat.2009.05.044
- Ottaviano, J. G., Cai, J., Murphy, R. S. (2014). Assessing the decontamination efficiency of a three-component flocculating system in the treatment of oilfield-produced water. Water Research, 52, 122–130. doi: https://doi.org/10.1016/j.watres.2014.01.004
- Nesrine, L., Salima, K., Lamine, K. M., Belaid, L., Souad, Bk., Lamine, G. M. et al. (2020). Phylogenetic characterization and screening of halophilic bacteria from Algerian salt lake for the production of biosurfactant and enzymes. World Journal of Biology and Biotechnology, 5 (2), 1. doi: https://doi.org/10.33865/wjb.005.02.0294
- Neff, J., Lee, K., DeBlois, E. M. (2011). Produced Water: Overview of Composition, Fates, and Effects. Produced Water, 3–54. doi: https://doi.org/10.1007/978-1-4614-0046-2_1
- Jiménez, S., Micó, M. M., Arnaldos, M., Medina, F., Contreras, S. (2018). State of the art of produced water treatment. Chemosphere, 192, 186–208. doi: https://doi.org/10.1016/j.chemosphere.2017.10.139
- Azmi, M. F., Soedarsono, J. W. (2018). Study of corrosion resistrance of pipeline API 5L X42 using green inhibitor bawang dayak (Eleutherine americanna Merr.) in 1M HCl. IOP Conference Series: Earth and Environmental Science, 105, 012061. doi: https://doi.org/10.1088/1755-1315/105/1/012061
- Arlan, A. S., Subekti, N., Soedarsono, J. W., Rustandi, A. (2018). Corrosion Inhibition by a Caesalpinia Sappan L Modified Imidazoline for Carbon Steel API 5L Grade X60 in HCl 1M Environment. Materials Science Forum, 929, 158–170. doi: https://doi.org/10.4028/www.scientific.net/msf.929.158
- Kaban, A. P. S., Ridhova, A., Priyotomo, G., Elya, B., Maksum, A., Sadeli, Y. et al. (2021). Development of white tea extract as green corrosion inhibitor in mild steel under 1 M hydrochloric acid solution. Eastern-European Journal of Enterprise Technologies, 2 (6 (110)), 6–20. doi: https://doi.org/10.15587/1729-4061.2021.224435
- Kaban, A., Mayangsari, W., Anwar, M., Maksum, A., Aditiyawarman, T., Soedarsono, J. et al. (2022). Unraveling the study of liquid smoke from rice husks as a green corrosion inhibitor in mild steel under 1 M HCl. Eastern-European Journal of Enterprise Technologies, 5 (6 (119)), 41–53. doi: https://doi.org/10.15587/1729-4061.2022.265086
- Gurjar, S., Sharma, S. K., Sharma, A., Ratnani, S. (2021). Performance of imidazolium based ionic liquids as corrosion inhibitors in acidic medium: A review. Applied Surface Science Advances, 6, 100170. doi: https://doi.org/10.1016/j.apsadv.2021.100170
- Mo, S., Li, L. J., Luo, H. Q., Li, N. B. (2017). An example of green copper corrosion inhibitors derived from flavor and medicine: Vanillin and isoniazid. Journal of Molecular Liquids, 242, 822–830. doi: https://doi.org/10.1016/j.molliq.2017.07.081
- Lu, H., Huang, K., Azimi, M., Guo, L. (2019). Blockchain Technology in the Oil and Gas Industry: A Review of Applications, Opportunities, Challenges, and Risks. IEEE Access, 7, 41426–41444. doi: https://doi.org/10.1109/access.2019.2907695
- Hasmila, I., Natsir, H., Soekamto, N. H. (2019). Phytochemical analysis and antioxidant activity of soursop leaf extract (Annona muricata Linn.). Journal of Physics: Conference Series, 1341 (3), 032027. doi: https://doi.org/10.1088/1742-6596/1341/3/032027
- Uwah, I. E., Okafor, P. C., Ebiekpe, V. E. (2013). Inhibitive action of ethanol extracts from Nauclea latifolia on the corrosion of mild steel in H2SO4 solutions and their adsorption characteristics. Arabian Journal of Chemistry, 6 (3), 285–293. doi: https://doi.org/10.1016/j.arabjc.2010.10.008
- Valdez-Salas, B., Vazquez-Delgado, R., Salvador-Carlos, J., Beltran-Partida, E., Salinas-Martinez, R., Cheng, N., Curiel-Alvarez, M. (2021). Azadirachta indica Leaf Extract as Green Corrosion Inhibitor for Reinforced Concrete Structures: Corrosion Effectiveness against Commercial Corrosion Inhibitors and Concrete Integrity. Materials, 14 (12), 3326. doi: https://doi.org/10.3390/ma14123326
- Saratha, R., Vasudha, V. G. (2010). Emblica Officinalis (Indian Gooseberry) Leaves Extract as Corrosion Inhibitor for Mild Steel in 1N HCl Medium. E-Journal of Chemistry, 7 (3), 677–684. doi: https://doi.org/10.1155/2010/162375
- Okafor, P. C., Uwah, I. E., Ekerenam, O. O., Ekpe, U. J. (2009). Combretum bracteosum extracts as eco‐friendly corrosion inhibitor for mild steel in acidic medium. Pigment & Resin Technology, 38 (4), 236–241. doi: https://doi.org/10.1108/03699420910973323
- Haldhar, R., Prasad, D., Bhardwaj, N. (2019). Extraction and experimental studies of Citrus aurantifolia as an economical and green corrosion inhibitor for mild steel in acidic media. Journal of Adhesion Science and Technology, 33 (11), 1169–1183. doi: https://doi.org/10.1080/01694243.2019.1585030
- Abdellattif, M. H., Alrefaee, S. H., Dagdag, O., Verma, C., Quraishi, M. A. (2021). Calotropis procera extract as an environmental friendly corrosion Inhibitor: Computational demonstrations. Journal of Molecular Liquids, 337, 116954. doi: https://doi.org/10.1016/j.molliq.2021.116954
- Widyastuti, D. A., Rahayu, P. (2017). Antioxidant Capacity Comparison of Ethanolic Extract of Soursop (Annona muricata Linn.) Leaves and Seeds as Cancer Prevention Candidate. Biology, Medicine, & Natural Product Chemistry, 6 (1), 1. doi: https://doi.org/10.14421/biomedich.2017.61.1-4
- Riastuti, R., Setiawidiani, D., Soedarsono, J. W., Aribowo, S., Kaban, A. P. S. (2022). Development of saga (Abrus precatorius) seed extract as a green corrosion inhibitor in API 5l Grade B under 1m HCL solutions. Eastern-European Journal of Enterprise Technologies, 4 (6 (118)), 46–56. doi: https://doi.org/10.15587/1729-4061.2022.263236
- Kaban, E. E., Maksum, A., Permana, S., Soedarsono, J. W. (2018). Utilization of secang heartwood (caesalpinia sappan l) as a green corrosion inhibitor on carbon steel (API 5L Gr. B) in 3.5% NaCl environment. IOP Conference Series: Earth and Environmental Science, 105, 012062. doi: https://doi.org/10.1088/1755-1315/105/1/012062
- Ayende, Rustandi, A., Soedarsono, J. W., Priadi, D., Sulistijono, Suprapta, D. N., Priyotomo, G., Bakri, R. (2014). Interaction of Purple Sweet Potato Extract with Ascorbic Acid in FeCl3 Solution. Applied Mechanics and Materials, 680, 32–37. doi: https://doi.org/10.4028/www.scientific.net/amm.680.32
- Xie, M. (2021). Castor-Bean Extract as an Inhibitor for Low Carbon Steel Corrosion in Simulated Oilfield Produced Water. International Journal of Electrochemical Science. doi: https://doi.org/10.20964/2021.08.24
- Standard Reference Test Method for Making Potentiostatic and Potentiodynamic Anodic Polarization Measurements (1994). ASTM.
- Zheng, Z., Hu, J., Eliaz, N., Zhou, L., Yuan, X., Zhong, X. (2022). Mercaptopropionic acid-modified oleic imidazoline as a highly efficient corrosion inhibitor for carbon steel in CO2-saturated formation water. Corrosion Science, 194, 109930. doi: https://doi.org/10.1016/j.corsci.2021.109930
- Chauhan, D. S., Quraishi, M. A., Srivastava, V., Haque, J., Ibrahimi, B. E. (2021). Virgin and chemically functionalized amino acids as green corrosion inhibitors: Influence of molecular structure through experimental and in silico studies. Journal of Molecular Structure, 1226, 129259. doi: https://doi.org/10.1016/j.molstruc.2020.129259
- Attou, A., Tourabi, M., Benikdes, A., Benali, O., Ouici, H. B., Benhiba, F. et al. (2020). Experimental studies and computational exploration on the 2-amino-5-(2-methoxyphenyl)-1,3,4-thiadiazole as novel corrosion inhibitor for mild steel in acidic environment. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 604, 125320. doi: https://doi.org/10.1016/j.colsurfa.2020.125320
- Chen, Z., Wang, M., Fadhil, A. A., Fu, C., Chen, T., Chen, M. et al. (2021). Preparation, characterization, and corrosion inhibition performance of graphene oxide quantum dots for Q235 steel in 1 M hydrochloric acid solution. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 627, 127209. doi: https://doi.org/10.1016/j.colsurfa.2021.127209
- Galai, M., Rbaa, M., Ouakki, M., Abousalem, A. S., Ech-chihbi, E., Dahmani, K. et al. (2020). Chemically functionalized of 8-hydroxyquinoline derivatives as efficient corrosion inhibition for steel in 1.0 M HCl solution: Experimental and theoretical studies. Surfaces and Interfaces, 21, 100695. doi: https://doi.org/10.1016/j.surfin.2020.100695
- Rekkab, S. et al. (2012). Green corrosion inhibitor from essential oil of eucalyptus globulus (Myrtaceae) for C38 steel in sulfuric acid solution. J. Mater. Environ. Sci., 3 (4), 613–627. Available at: https://www.jmaterenvironsci.com/Document/vol3/vol3_N4/61-JMES-269-2012-Rekkab.pdf
- Paul Setiawan Kaban, A., Mayangsari, W., Syaiful Anwar, M., Maksum, A., Riastuti, R., Aditiyawarman, T., Wahyuadi Soedarsono, J. (2022). Experimental and modelling waste rice husk ash as a novel green corrosion inhibitor under acidic environment. Materials Today: Proceedings, 62, 4225–4234. doi: https://doi.org/10.1016/j.matpr.2022.04.738
- Melo, T., Figueiredo, A. R. P., da Costa, E., Couto, D., Silva, J., Domingues, M. R., Domingues, P. (2021). Ethanol Extraction of Polar Lipids from Nannochloropsis oceanica for Food, Feed, and Biotechnology Applications Evaluated Using Lipidomic Approaches. Marine Drugs, 19 (11), 593. doi: https://doi.org/10.3390/md19110593
- Tourabi, M., Nohair, K., Traisnel, M., Jama, C., Bentiss, F. (2013). Electrochemical and XPS studies of the corrosion inhibition of carbon steel in hydrochloric acid pickling solutions by 3,5-bis(2-thienylmethyl)-4-amino-1,2,4-triazole. Corrosion Science, 75, 123–133. doi: https://doi.org/10.1016/j.corsci.2013.05.023
- Baux, J., Caussé, N., Esvan, J., Delaunay, S., Tireau, J., Roy, M. et al. (2018). Impedance analysis of film-forming amines for the corrosion protection of a carbon steel. Electrochimica Acta, 283, 699–707. doi: https://doi.org/10.1016/j.electacta.2018.06.189
- Chowdhury, M. A., Ahmed, M. M. S., Hossain, N., Islam, M. A., Islam, S., Rana, M. M. (2023). Tulsi and green tea extracts as efficient green corrosion inhibitor for the corrosion of aluminum alloy in acidic medium. Results in Engineering, 17, 100996. doi: https://doi.org/10.1016/j.rineng.2023.100996
- Vasyliev, G. S., Vorobyova, V. I., Linyucheva, O. V. (2020). Evaluation of Reducing Ability and Antioxidant Activity of Fruit Pomace Extracts by Spectrophotometric and Electrochemical Methods. Journal of Analytical Methods in Chemistry, 2020, 1–16. doi: https://doi.org/10.1155/2020/8869436
- Bhardwaj, N., Sharma, P., Kumar, V. (2021). Phytochemicals as steel corrosion inhibitor: an insight into mechanism. Corrosion Reviews, 39 (1), 27–41. doi: https://doi.org/10.1515/corrrev-2020-0046
- Yaro, A. S., Khadom, A. A., Wael, R. K. (2013). Apricot juice as green corrosion inhibitor of mild steel in phosphoric acid. Alexandria Engineering Journal, 52 (1), 129–135. doi: https://doi.org/10.1016/j.aej.2012.11.001
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2023 Ayende, Rini Riastuti, Johny Wahyuadi Soedarsono, Agus Paul Setiawan Kaban, Mohammad Ikbal Hikmawan, Rizal Tresna Rahmdani

This work is licensed under a Creative Commons Attribution 4.0 International License.
The consolidation and conditions for the transfer of copyright (identification of authorship) is carried out in the License Agreement. In particular, the authors reserve the right to the authorship of their manuscript and transfer the first publication of this work to the journal under the terms of the Creative Commons CC BY license. At the same time, they have the right to conclude on their own additional agreements concerning the non-exclusive distribution of the work in the form in which it was published by this journal, but provided that the link to the first publication of the article in this journal is preserved.
A license agreement is a document in which the author warrants that he/she owns all copyright for the work (manuscript, article, etc.).
The authors, signing the License Agreement with TECHNOLOGY CENTER PC, have all rights to the further use of their work, provided that they link to our edition in which the work was published.
According to the terms of the License Agreement, the Publisher TECHNOLOGY CENTER PC does not take away your copyrights and receives permission from the authors to use and dissemination of the publication through the world's scientific resources (own electronic resources, scientometric databases, repositories, libraries, etc.).
In the absence of a signed License Agreement or in the absence of this agreement of identifiers allowing to identify the identity of the author, the editors have no right to work with the manuscript.
It is important to remember that there is another type of agreement between authors and publishers – when copyright is transferred from the authors to the publisher. In this case, the authors lose ownership of their work and may not use it in any way.








