Розробка Annona muricata Linn в якості зеленого інгібітора корозії у пластовій воді: ефективність інгібування та модель адсорбції
DOI:
https://doi.org/10.15587/1729-4061.2023.278911Ключові слова:
зелені інгібітори корозії, органічні інгібітори корозії, Annona muricata Linn, адсорбційне інгібування аннони колючоїАнотація
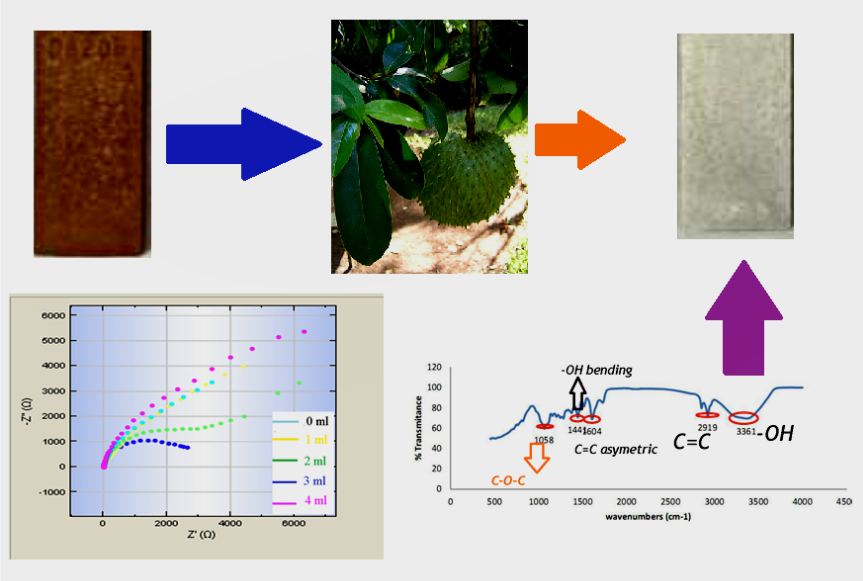
У даній роботі аннона колюча використовувалася в якості зеленого інгібітора корозії для захисту сталі API 5L марки A від шкідливого впливу корозії у пластовій воді. Незважаючи на ефективність неорганічних інгібіторів, останні дані їх випробувань на токсичність показують, що застосування органічних інгібіторів є суттєвим для заміни синтетичних інгібіторів корозії. Однак використання аннони колючої в якості зеленого інгібітора корозії погано вивчено через відсутність комплексного способу екстракції та механізму інгібування. Для виявлення природи інгібування корозії було проведено кілька випробувань, включаючи визначення втрати маси, потенціодинамічну поляризацію та електрохімічну імпедансну спектроскопію (EIS). За допомогою інфрачервоної спектроскопії з перетворенням Фур'є виявлено зв'язування домінуючих функціональних груп з субстратом. Результати потенціодинамічної поляризації показують, що інгібітор є інгібітором змішаного типу, що впливає на анодну та катодну реакції. Випробування на втрату маси демонструє найвищу ефективність інгібування 52,62 % при додаванні 2 мл інгібіторів протягом восьми днів спостереження. Результати поляризації та EIS показують, що інгібітор знижує швидкість корозії з більш високим показником інгібування 88,52 %. Зазначений результат пов'язаний з приєднанням неполярних та полярних функціональних груп Annona muricata Linn. Основна функціональна група включає в себе C=O, C‒C та –O. H., які активно пов'язані з поверхнею металу. Ароматична група при хвильовому числі 1050 і 1090 см-1 показує присутність ефіру і виступає в якості центру адсорбції. У даній роботі поєднання трьох розчинників, гексану, ацетону і етанолу, забезпечує повну екстракцію переважаючої сполуки з аннони колючої
Посилання
- Liu, H., Gu, T., Zhang, G., Wang, W., Dong, S., Cheng, Y., Liu, H. (2016). Corrosion inhibition of carbon steel in CO2-containing oilfield produced water in the presence of iron-oxidizing bacteria and inhibitors. Corrosion Science, 105, 149–160. doi: https://doi.org/10.1016/j.corsci.2016.01.012
- Verma, C., Ebenso, E. E., Bahadur, I., Quraishi, M. A. (2018). An overview on plant extracts as environmental sustainable and green corrosion inhibitors for metals and alloys in aggressive corrosive media. Journal of Molecular Liquids, 266, 577–590. doi: https://doi.org/10.1016/j.molliq.2018.06.110
- Fakhru’l-Razi, A., Pendashteh, A., Abdullah, L. C., Biak, D. R. A., Madaeni, S. S., Abidin, Z. Z. (2009). Review of technologies for oil and gas produced water treatment. Journal of Hazardous Materials, 170 (2-3), 530–551. doi: https://doi.org/10.1016/j.jhazmat.2009.05.044
- Ottaviano, J. G., Cai, J., Murphy, R. S. (2014). Assessing the decontamination efficiency of a three-component flocculating system in the treatment of oilfield-produced water. Water Research, 52, 122–130. doi: https://doi.org/10.1016/j.watres.2014.01.004
- Nesrine, L., Salima, K., Lamine, K. M., Belaid, L., Souad, Bk., Lamine, G. M. et al. (2020). Phylogenetic characterization and screening of halophilic bacteria from Algerian salt lake for the production of biosurfactant and enzymes. World Journal of Biology and Biotechnology, 5 (2), 1. doi: https://doi.org/10.33865/wjb.005.02.0294
- Neff, J., Lee, K., DeBlois, E. M. (2011). Produced Water: Overview of Composition, Fates, and Effects. Produced Water, 3–54. doi: https://doi.org/10.1007/978-1-4614-0046-2_1
- Jiménez, S., Micó, M. M., Arnaldos, M., Medina, F., Contreras, S. (2018). State of the art of produced water treatment. Chemosphere, 192, 186–208. doi: https://doi.org/10.1016/j.chemosphere.2017.10.139
- Azmi, M. F., Soedarsono, J. W. (2018). Study of corrosion resistrance of pipeline API 5L X42 using green inhibitor bawang dayak (Eleutherine americanna Merr.) in 1M HCl. IOP Conference Series: Earth and Environmental Science, 105, 012061. doi: https://doi.org/10.1088/1755-1315/105/1/012061
- Arlan, A. S., Subekti, N., Soedarsono, J. W., Rustandi, A. (2018). Corrosion Inhibition by a Caesalpinia Sappan L Modified Imidazoline for Carbon Steel API 5L Grade X60 in HCl 1M Environment. Materials Science Forum, 929, 158–170. doi: https://doi.org/10.4028/www.scientific.net/msf.929.158
- Kaban, A. P. S., Ridhova, A., Priyotomo, G., Elya, B., Maksum, A., Sadeli, Y. et al. (2021). Development of white tea extract as green corrosion inhibitor in mild steel under 1 M hydrochloric acid solution. Eastern-European Journal of Enterprise Technologies, 2 (6 (110)), 6–20. doi: https://doi.org/10.15587/1729-4061.2021.224435
- Kaban, A., Mayangsari, W., Anwar, M., Maksum, A., Aditiyawarman, T., Soedarsono, J. et al. (2022). Unraveling the study of liquid smoke from rice husks as a green corrosion inhibitor in mild steel under 1 M HCl. Eastern-European Journal of Enterprise Technologies, 5 (6 (119)), 41–53. doi: https://doi.org/10.15587/1729-4061.2022.265086
- Gurjar, S., Sharma, S. K., Sharma, A., Ratnani, S. (2021). Performance of imidazolium based ionic liquids as corrosion inhibitors in acidic medium: A review. Applied Surface Science Advances, 6, 100170. doi: https://doi.org/10.1016/j.apsadv.2021.100170
- Mo, S., Li, L. J., Luo, H. Q., Li, N. B. (2017). An example of green copper corrosion inhibitors derived from flavor and medicine: Vanillin and isoniazid. Journal of Molecular Liquids, 242, 822–830. doi: https://doi.org/10.1016/j.molliq.2017.07.081
- Lu, H., Huang, K., Azimi, M., Guo, L. (2019). Blockchain Technology in the Oil and Gas Industry: A Review of Applications, Opportunities, Challenges, and Risks. IEEE Access, 7, 41426–41444. doi: https://doi.org/10.1109/access.2019.2907695
- Hasmila, I., Natsir, H., Soekamto, N. H. (2019). Phytochemical analysis and antioxidant activity of soursop leaf extract (Annona muricata Linn.). Journal of Physics: Conference Series, 1341 (3), 032027. doi: https://doi.org/10.1088/1742-6596/1341/3/032027
- Uwah, I. E., Okafor, P. C., Ebiekpe, V. E. (2013). Inhibitive action of ethanol extracts from Nauclea latifolia on the corrosion of mild steel in H2SO4 solutions and their adsorption characteristics. Arabian Journal of Chemistry, 6 (3), 285–293. doi: https://doi.org/10.1016/j.arabjc.2010.10.008
- Valdez-Salas, B., Vazquez-Delgado, R., Salvador-Carlos, J., Beltran-Partida, E., Salinas-Martinez, R., Cheng, N., Curiel-Alvarez, M. (2021). Azadirachta indica Leaf Extract as Green Corrosion Inhibitor for Reinforced Concrete Structures: Corrosion Effectiveness against Commercial Corrosion Inhibitors and Concrete Integrity. Materials, 14 (12), 3326. doi: https://doi.org/10.3390/ma14123326
- Saratha, R., Vasudha, V. G. (2010). Emblica Officinalis (Indian Gooseberry) Leaves Extract as Corrosion Inhibitor for Mild Steel in 1N HCl Medium. E-Journal of Chemistry, 7 (3), 677–684. doi: https://doi.org/10.1155/2010/162375
- Okafor, P. C., Uwah, I. E., Ekerenam, O. O., Ekpe, U. J. (2009). Combretum bracteosum extracts as eco‐friendly corrosion inhibitor for mild steel in acidic medium. Pigment & Resin Technology, 38 (4), 236–241. doi: https://doi.org/10.1108/03699420910973323
- Haldhar, R., Prasad, D., Bhardwaj, N. (2019). Extraction and experimental studies of Citrus aurantifolia as an economical and green corrosion inhibitor for mild steel in acidic media. Journal of Adhesion Science and Technology, 33 (11), 1169–1183. doi: https://doi.org/10.1080/01694243.2019.1585030
- Abdellattif, M. H., Alrefaee, S. H., Dagdag, O., Verma, C., Quraishi, M. A. (2021). Calotropis procera extract as an environmental friendly corrosion Inhibitor: Computational demonstrations. Journal of Molecular Liquids, 337, 116954. doi: https://doi.org/10.1016/j.molliq.2021.116954
- Widyastuti, D. A., Rahayu, P. (2017). Antioxidant Capacity Comparison of Ethanolic Extract of Soursop (Annona muricata Linn.) Leaves and Seeds as Cancer Prevention Candidate. Biology, Medicine, & Natural Product Chemistry, 6 (1), 1. doi: https://doi.org/10.14421/biomedich.2017.61.1-4
- Riastuti, R., Setiawidiani, D., Soedarsono, J. W., Aribowo, S., Kaban, A. P. S. (2022). Development of saga (Abrus precatorius) seed extract as a green corrosion inhibitor in API 5l Grade B under 1m HCL solutions. Eastern-European Journal of Enterprise Technologies, 4 (6 (118)), 46–56. doi: https://doi.org/10.15587/1729-4061.2022.263236
- Kaban, E. E., Maksum, A., Permana, S., Soedarsono, J. W. (2018). Utilization of secang heartwood (caesalpinia sappan l) as a green corrosion inhibitor on carbon steel (API 5L Gr. B) in 3.5% NaCl environment. IOP Conference Series: Earth and Environmental Science, 105, 012062. doi: https://doi.org/10.1088/1755-1315/105/1/012062
- Ayende, Rustandi, A., Soedarsono, J. W., Priadi, D., Sulistijono, Suprapta, D. N., Priyotomo, G., Bakri, R. (2014). Interaction of Purple Sweet Potato Extract with Ascorbic Acid in FeCl3 Solution. Applied Mechanics and Materials, 680, 32–37. doi: https://doi.org/10.4028/www.scientific.net/amm.680.32
- Xie, M. (2021). Castor-Bean Extract as an Inhibitor for Low Carbon Steel Corrosion in Simulated Oilfield Produced Water. International Journal of Electrochemical Science. doi: https://doi.org/10.20964/2021.08.24
- Standard Reference Test Method for Making Potentiostatic and Potentiodynamic Anodic Polarization Measurements (1994). ASTM.
- Zheng, Z., Hu, J., Eliaz, N., Zhou, L., Yuan, X., Zhong, X. (2022). Mercaptopropionic acid-modified oleic imidazoline as a highly efficient corrosion inhibitor for carbon steel in CO2-saturated formation water. Corrosion Science, 194, 109930. doi: https://doi.org/10.1016/j.corsci.2021.109930
- Chauhan, D. S., Quraishi, M. A., Srivastava, V., Haque, J., Ibrahimi, B. E. (2021). Virgin and chemically functionalized amino acids as green corrosion inhibitors: Influence of molecular structure through experimental and in silico studies. Journal of Molecular Structure, 1226, 129259. doi: https://doi.org/10.1016/j.molstruc.2020.129259
- Attou, A., Tourabi, M., Benikdes, A., Benali, O., Ouici, H. B., Benhiba, F. et al. (2020). Experimental studies and computational exploration on the 2-amino-5-(2-methoxyphenyl)-1,3,4-thiadiazole as novel corrosion inhibitor for mild steel in acidic environment. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 604, 125320. doi: https://doi.org/10.1016/j.colsurfa.2020.125320
- Chen, Z., Wang, M., Fadhil, A. A., Fu, C., Chen, T., Chen, M. et al. (2021). Preparation, characterization, and corrosion inhibition performance of graphene oxide quantum dots for Q235 steel in 1 M hydrochloric acid solution. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 627, 127209. doi: https://doi.org/10.1016/j.colsurfa.2021.127209
- Galai, M., Rbaa, M., Ouakki, M., Abousalem, A. S., Ech-chihbi, E., Dahmani, K. et al. (2020). Chemically functionalized of 8-hydroxyquinoline derivatives as efficient corrosion inhibition for steel in 1.0 M HCl solution: Experimental and theoretical studies. Surfaces and Interfaces, 21, 100695. doi: https://doi.org/10.1016/j.surfin.2020.100695
- Rekkab, S. et al. (2012). Green corrosion inhibitor from essential oil of eucalyptus globulus (Myrtaceae) for C38 steel in sulfuric acid solution. J. Mater. Environ. Sci., 3 (4), 613–627. Available at: https://www.jmaterenvironsci.com/Document/vol3/vol3_N4/61-JMES-269-2012-Rekkab.pdf
- Paul Setiawan Kaban, A., Mayangsari, W., Syaiful Anwar, M., Maksum, A., Riastuti, R., Aditiyawarman, T., Wahyuadi Soedarsono, J. (2022). Experimental and modelling waste rice husk ash as a novel green corrosion inhibitor under acidic environment. Materials Today: Proceedings, 62, 4225–4234. doi: https://doi.org/10.1016/j.matpr.2022.04.738
- Melo, T., Figueiredo, A. R. P., da Costa, E., Couto, D., Silva, J., Domingues, M. R., Domingues, P. (2021). Ethanol Extraction of Polar Lipids from Nannochloropsis oceanica for Food, Feed, and Biotechnology Applications Evaluated Using Lipidomic Approaches. Marine Drugs, 19 (11), 593. doi: https://doi.org/10.3390/md19110593
- Tourabi, M., Nohair, K., Traisnel, M., Jama, C., Bentiss, F. (2013). Electrochemical and XPS studies of the corrosion inhibition of carbon steel in hydrochloric acid pickling solutions by 3,5-bis(2-thienylmethyl)-4-amino-1,2,4-triazole. Corrosion Science, 75, 123–133. doi: https://doi.org/10.1016/j.corsci.2013.05.023
- Baux, J., Caussé, N., Esvan, J., Delaunay, S., Tireau, J., Roy, M. et al. (2018). Impedance analysis of film-forming amines for the corrosion protection of a carbon steel. Electrochimica Acta, 283, 699–707. doi: https://doi.org/10.1016/j.electacta.2018.06.189
- Chowdhury, M. A., Ahmed, M. M. S., Hossain, N., Islam, M. A., Islam, S., Rana, M. M. (2023). Tulsi and green tea extracts as efficient green corrosion inhibitor for the corrosion of aluminum alloy in acidic medium. Results in Engineering, 17, 100996. doi: https://doi.org/10.1016/j.rineng.2023.100996
- Vasyliev, G. S., Vorobyova, V. I., Linyucheva, O. V. (2020). Evaluation of Reducing Ability and Antioxidant Activity of Fruit Pomace Extracts by Spectrophotometric and Electrochemical Methods. Journal of Analytical Methods in Chemistry, 2020, 1–16. doi: https://doi.org/10.1155/2020/8869436
- Bhardwaj, N., Sharma, P., Kumar, V. (2021). Phytochemicals as steel corrosion inhibitor: an insight into mechanism. Corrosion Reviews, 39 (1), 27–41. doi: https://doi.org/10.1515/corrrev-2020-0046
- Yaro, A. S., Khadom, A. A., Wael, R. K. (2013). Apricot juice as green corrosion inhibitor of mild steel in phosphoric acid. Alexandria Engineering Journal, 52 (1), 129–135. doi: https://doi.org/10.1016/j.aej.2012.11.001
##submission.downloads##
Опубліковано
Як цитувати
Номер
Розділ
Ліцензія
Авторське право (c) 2023 Ayende, Rini Riastuti, Johny Wahyuadi Soedarsono, Agus Paul Setiawan Kaban, Mohammad Ikbal Hikmawan, Rizal Tresna Rahmdani

Ця робота ліцензується відповідно до Creative Commons Attribution 4.0 International License.
Закріплення та умови передачі авторських прав (ідентифікація авторства) здійснюється у Ліцензійному договорі. Зокрема, автори залишають за собою право на авторство свого рукопису та передають журналу право першої публікації цієї роботи на умовах ліцензії Creative Commons CC BY. При цьому вони мають право укладати самостійно додаткові угоди, що стосуються неексклюзивного поширення роботи у тому вигляді, в якому вона була опублікована цим журналом, але за умови збереження посилання на першу публікацію статті в цьому журналі.
Ліцензійний договір – це документ, в якому автор гарантує, що володіє усіма авторськими правами на твір (рукопис, статтю, тощо).
Автори, підписуючи Ліцензійний договір з ПП «ТЕХНОЛОГІЧНИЙ ЦЕНТР», мають усі права на подальше використання свого твору за умови посилання на наше видання, в якому твір опублікований. Відповідно до умов Ліцензійного договору, Видавець ПП «ТЕХНОЛОГІЧНИЙ ЦЕНТР» не забирає ваші авторські права та отримує від авторів дозвіл на використання та розповсюдження публікації через світові наукові ресурси (власні електронні ресурси, наукометричні бази даних, репозитарії, бібліотеки тощо).
За відсутності підписаного Ліцензійного договору або за відсутністю вказаних в цьому договорі ідентифікаторів, що дають змогу ідентифікувати особу автора, редакція не має права працювати з рукописом.
Важливо пам’ятати, що існує і інший тип угоди між авторами та видавцями – коли авторські права передаються від авторів до видавця. В такому разі автори втрачають права власності на свій твір та не можуть його використовувати в будь-який спосіб.









