Розробка комплексної технології очищення промислових залізосульфатвмісних стоків гальванічного виробництва
DOI:
https://doi.org/10.15587/1729-4061.2023.291383Ключові слова:
електродіаліз, гальванічні стоки, залізосульфатвмісні розчини, трикамерний електролізер, феритний методАнотація
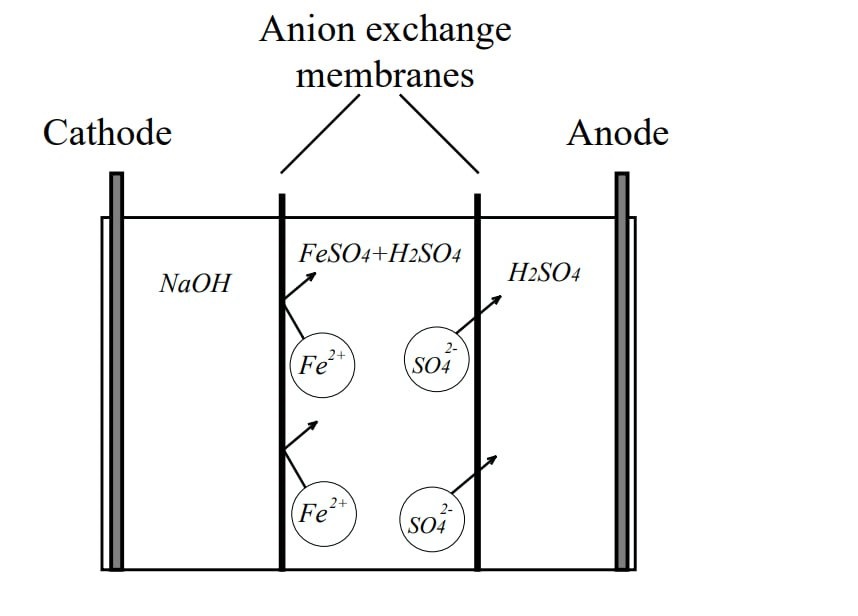
Об’єктом дослідження є комплексне електрохімічне та феритне знешкодження залізосульфатвмісних вод. Переробка рідких відходів здійснюється шляхом електрохімічної обробки із застосуванням дво- та трикамерних електролізерів. В роботі досліджено процеси електродіалізного очищення імітаційних розчинів з концентраціями сполук FeSO4 5 г/дм3 та Н2SO4 300–2100 мг-екв/дм3. В якості катода використано пластину із нержавіючої сталі, в якості аноду – пластини з титану, вкритого оксидом рутенію, та свинцю. Показано, що найвищий вихід за струмом продуктів електродіалізу 84,5 % отриманий при застосуванні трикамерного електролізера з аніонообмінними мембранами МА-41. Встановлено, що при застосуванні вказаного електролізера суттєвий вплив на процес розділення домішок чинить градієнт концентрації, величина якого прямо пропорційна різниці концентрацій вихідних розчинів, якими заповнені приелектродні камери. Показано, що для двокамерного електролізера вихід за струмом сягає 72 %, що поясняється шкідливим впливом значного градієнту концентрацій та проявляється в швидкому механічному блокуванні мембрани і сповільненні процесу міграції іонів, а також зростанні енерговитрат. В двокамерному електролізері отримано Н2SO4 з концентрацією 18,3 %, яка придатна для повторного використання в ваннах травлення. З’ясовано, що в результаті електродіалізного розділення і додаткового окиснення концентровані залізосульфатні розчини доцільно застосовувати для одержання феритного матеріалу кристалічної структури з розмірами частинок 2–20 мкм. В рамках циркуляційної економіки запропоновано екологічно безпечну технологію знешкодження промислових залізовмісних сульфатних розчинів гальванічного виробництва з використанням комплексу електродіалізного та феритного методів
Посилання
- Korchemlyuk, M., Arkhipova, L., Kravchynskyi, R. L., Mykhailyuk, J. D. (2019). Anthropogenic influence from point and diffuse sources of pollution in the Upper Prut River basin. Naukovyi Visnyk Natsionalnoho Hirnychoho Universytetu, 1, 125–131. doi: https://doi.org/10.29202/nvngu/2019-1/12
- Monastyrov, M., Prikhna, T., Halbedel, B., Kochetov, G., Marquis, F. D. S., Mamalis, A. G., Prysiazhna, O. (2019). Electroerosion dispersion, sorption and coagulation for complex water purification: Electroerosion waste recycling and manufacturing of metal, oxide and alloy nanopowders. Nanotechnology Perceptions, 15 (1), 48–57. doi: https://doi.org/10.4024/n24mo18a.ntp.15.01
- Custodio, M., Peñaloza, R. (2021). Evaluation of the Distribution of Heavy Metals and Arsenic in Inland Wetlands (Peru) Using Multivariate Statistical Methods. Ecological Engineering & Environmental Technology, 22 (3), 104–111. doi: https://doi.org/10.12912/27197050/135522
- Frolova, L. (2019). Using of spent etching solution to obtain yellow ferric oxide pigments. Modern Problems of Metalurgy, 1 (21), 82–86. doi: https://doi.org/10.34185/1991-7848.2018.01.13
- Environment of Ukraine 2020. Statistical Publication. State Statistics Service of Ukraine. Available at: https://ukrstat.gov.ua/druk/publicat/kat_u/2021/zb/11/Dovk_20.pdf
- Natsionalna dopovid pro stan navkolyshnoho pryrodnoho seredovyshcha v Ukraini u 2021 rotsi. Ministerstvo zakhystu dovkillia ta pryrodnykh resursiv Ukrainy. Available at: https://mepr.gov.ua/wp-content/uploads/2023/01/Natsdopovid-2021-n.pdf
- Dvostoronnie spivrobitnytstvo u sferi upravlinnia vodnymy resursamy. Derzhavne ahentstvo vodnykh resursiv Ukrainy. Available at: https://davr.gov.ua/transkordonne-spivrobitnictvo
- Shabliy, T., Gomelya, M., Kryzhanovska, Y., Levytska, O. (2020). Utilization of Sodium Chloride Solutions to Obtain Ferrous Chlorides. Journal of Ecological Engineering, 21 (8), 177–184. doi: https://doi.org/10.12911/22998993/126966
- Trus, I., Radovenchyk, I., Halysh, V., Chuprinov, E., Benatov, D., Hlushko, O., Sirenko, L. (2022). Innovative Method for Water Deiron Ions Using Capillary Material. Journal of Ecological Engineering, 23 (3), 174–182. doi: https://doi.org/10.12911/22998993/145467
- Ivanenko, O., Radovenchyk, V., Radovenchyk, І. (2020). Neutralization of carbon monoxide by magnetite-based catalysts. Technology Audit and Production Reserves, 5 (3 (55)), 24–28. doi: https://doi.org/10.15587/2706-5448.2020.214432
- Khokhotva, O., Butchenko, L., Gomelya, N. (2018). The use of modified and composite ferritic sorbents for selective extraction of Cu2+. Technical Sciences and Technology, 1 (11), 264–272. doi: https://doi.org/10.25140/2411-5363-2018-1(11)-264-272
- Kochetov, G., Samchenko, D., Kolodko, A., Kovalchuk, O., Pasko, A. (2018). Development of technology of industrial wastes treatment products disposal by ferritization in the matrix of alkali-activated cements. Technology Audit and Production Reserves, 6 (3 (44)), 31–35. doi: https://doi.org/10.15587/2312-8372.2018.152615
- Trus, I., Radovenchyk, I., Halysh, V., Skiba, M., Vasylenko, I., Vorobyova, V. et al. (2019). Innovative Approach in Creation of Integrated Technology of Desalination of Mineralized Water. Journal of Ecological Engineering, 20 (8), 107–113. doi: https://doi.org/10.12911/22998993/110767
- Gomelya, N., Hrabitchenko, V., Trohimennko, A., Shablij, T. (2016). Research into ion exchange softening of highly mineralized waters. Eastern-European Journal of Enterprise Technologies, 4 (10 (82)), 4. doi: https://doi.org/10.15587/1729-4061.2016.75338
- Akhter, M., Habib, G., Qamar, S. U. (2018). Application of Electrodialysis in Waste Water Treatment and Impact of Fouling on Process Performance. Journal of Membrane Science & Technology, 08 (02). doi: https://doi.org/10.4172/2155-9589.1000182
- Shabliy, T., Ivanenko, O., Plashykhin, S., Pavliuk, N., Safiants, A., Sidorov, D. (2023). New Approaches to Comprehensive Electrochemical Processing of Sulfate-Chloride High-Mineralized Wastewater Treatment Residues. Architecture, Civil Engineering, Environment, 16 (3), 171–180. doi: https://doi.org/10.2478/acee-2023-0044
- Radovenchyk, V. M., Ivanenko, O. I., Radovenchyk, Ya. V., Krysenko, T. V. (2020). Zastosuvannia ferytnykh materialiv v protsesakh ochyshchennia vody. Bila Tserkva: Vydavnytstvo O. V. Pshonkivskyi, 215. Available at: https://eco-paper.kpi.ua/CONTENT/literatyra/ferity_mono.pdf
- Samchenko, D. N., Kochetov, G. М., Vasiliev, A., Derecha, D. A., Skirta, Y. B., Lastivka, O. V. (2022). Energy-saving technology for processing of exhausted etching solutions with obtaining of ferromagnetic compounds. Environmental Safety and Natural Resources, 43 (3), 22–34. doi: https://doi.org/10.32347/2411-4049.2022.3.22-34
- Yemchura, B., Kochetov, G., Samchenko, D., Pakhomov, D., Puzanov, A. (2023). Study of the kinetics of the extraction of zinc ions from wastewater by ferritization. Problems of Water Supply, Sewerage and Hydraulic, 42, 13–18. doi: https://doi.org/10.32347/2524-0021.2023.42.13-18
- Kochetov, G., Prikhna, T., Kovalchuk, O., Samchenko, D. (2018). Research of the treatment of depleted nickelplating electrolytes by the ferritization method. Eastern-European Journal of Enterprise Technologies, 3 (6 (93)), 52–60. doi: https://doi.org/10.15587/1729-4061.2018.133797
- Gomelya, M., Shabliy, T., Radovenchyk, I., Overchenko, T., Halysh, V. (2019). Estimation of the Efficiency of Ammonia Oxidation in Anolyte of Two-Chamber Electrolyzer. Journal of Ecological Engineering, 20 (5), 121–129. doi: https://doi.org/10.12911/22998993/105337
- Melnyk, L., Goncharuk, V. (2009). Electrodialysis of solutions containing Mn (II) ions. Desalination, 241 (1-3), 49–56. doi: https://doi.org/10.1016/j.desal.2007.11.082
- Nabyvanets, B. Y., Osadchyi, V. I., Osadcha, N. M., Nabyvanets, Yu. B. (2007). Analitychna khimiya poverkhnevykh vod. Kyiv: Naukova dumka, 456. Available at: https://www.nas.gov.ua/UA/Book/Pages/default.aspx?BookID=0000002073
- Mane, R. S., Jadhav, V. V. (Eds.) (2020). Spinel Ferrite Nanostructures for Energy Storage Devices. Elsevier. doi: https://doi.org/10.1016/c2018-0-04420-5
- Kefeni, K. K., Msagati, T. A. M., Mamba, B. B. (2017). Ferrite nanoparticles: Synthesis, characterisation and applications in electronic device. Materials Science and Engineering: B, 215, 37–55. doi: https://doi.org/10.1016/j.mseb.2016.11.002
- Chkavro, Z., Antoniuk, N. (2014). Theory and practice of coagulant application in water treatment technology. Naukovi zapysky NaUKMA. Khimichni nauky i tekhnolohiyi, 157, 65–78. Available at: http://nbuv.gov.ua/UJRN/NaUKMAchem_2014_157_13
- Zlobin, I. O., Zubrychev, L. S. (2009). Perevahy novykh zalizovmisnykh koahuliantiv. Haluzeve mashynobuduvannia, budivnytstvo, 2. Available at: https://reposit.nupp.edu.ua/bitstream/PoltNTU/8283/1/Znpgmb_2009_2_35.pdf
- Council Directive 96/61/EC of 24 September 1996 concerning integrated pollution prevention and control. Available at: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A31996L0061
##submission.downloads##
Опубліковано
Як цитувати
Номер
Розділ
Ліцензія
Авторське право (c) 2023 Serhii Dovholap, Nikolai Gomelya, Olena Ivanenko, Svetlana Frolenkova, Tatiana Shabliy

Ця робота ліцензується відповідно до Creative Commons Attribution 4.0 International License.
Закріплення та умови передачі авторських прав (ідентифікація авторства) здійснюється у Ліцензійному договорі. Зокрема, автори залишають за собою право на авторство свого рукопису та передають журналу право першої публікації цієї роботи на умовах ліцензії Creative Commons CC BY. При цьому вони мають право укладати самостійно додаткові угоди, що стосуються неексклюзивного поширення роботи у тому вигляді, в якому вона була опублікована цим журналом, але за умови збереження посилання на першу публікацію статті в цьому журналі.
Ліцензійний договір – це документ, в якому автор гарантує, що володіє усіма авторськими правами на твір (рукопис, статтю, тощо).
Автори, підписуючи Ліцензійний договір з ПП «ТЕХНОЛОГІЧНИЙ ЦЕНТР», мають усі права на подальше використання свого твору за умови посилання на наше видання, в якому твір опублікований. Відповідно до умов Ліцензійного договору, Видавець ПП «ТЕХНОЛОГІЧНИЙ ЦЕНТР» не забирає ваші авторські права та отримує від авторів дозвіл на використання та розповсюдження публікації через світові наукові ресурси (власні електронні ресурси, наукометричні бази даних, репозитарії, бібліотеки тощо).
За відсутності підписаного Ліцензійного договору або за відсутністю вказаних в цьому договорі ідентифікаторів, що дають змогу ідентифікувати особу автора, редакція не має права працювати з рукописом.
Важливо пам’ятати, що існує і інший тип угоди між авторами та видавцями – коли авторські права передаються від авторів до видавця. В такому разі автори втрачають права власності на свій твір та не можуть його використовувати в будь-який спосіб.









