Evaluation of quality characteristics of fermented acidophilic product with B. Bifidum and Prunus padus extract
DOI:
https://doi.org/10.15587/1729-4061.2024.300373Keywords:
lactobacillus acidophilus, bifidobacterium bifidum, prebiotics, bird cherry extract, dairy productAbstract
Functional foods containing probiotics and/or prebiotics are of scientific and practical importance. The method of pre-activating bifidobacteria before their use in the production of fermented milk products has a profound impact on enhancing the quality of the resulting products. Finding ways to shorten the activation time of bifidobacteria in the production of functional foods with probiotics is an urgent task.
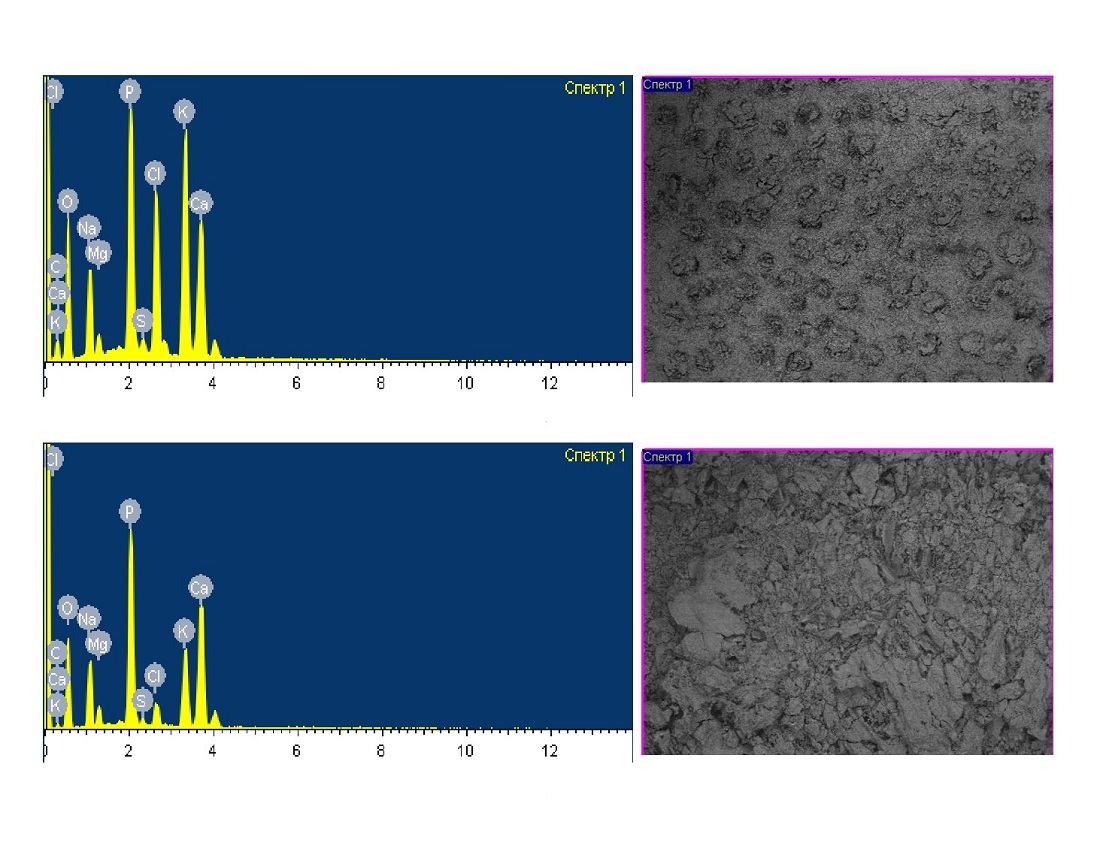
Shortening the activation time and optimizing enzyme systems of bifidobacteria with antioxidants are crucial for innovative probiotic fermented milk technology. The object of this research is the technology of fermented acidophilic products enriched with bifidobacteria activated using Prunus padus (bird cherry) extract, known for its antioxidant properties. The finished product showed a significant increase in bifidobacteria count, reaching 1×109 CFU, and a 25.7 % boost in L. acidophilus after 7 days.
Furthermore, the activation of bifidobacteria by Prunus padus extract resulted in a threefold increase in the histidine content and increased the content of oleic, eicosanoic, linoleic, arachidonic, and docosahexaenoic acids by 10.0 %, 26.4 %, 14.4 %, 22.6 %, and 66.6 % in the experimental sample compared to the control sample, respectively. Moreover, pentadecanoic, selacholeic, eicosatrienoic acids and tyrosine were present in the experimental but not in the control sample. Microbiological safety tests found no pathogenic microorganisms in the fermented acidophilic product, yet lactic acid microorganism levels exceeded norms in the experimental sample, confirming the product's probiotic properties and high physiological value. Thus, the developed product is characterized by better taste, a longer shelf life, and the preservation of bacterial titers
References
- Fijan, S. (2014). Microorganisms with Claimed Probiotic Properties: An Overview of Recent Literature. International Journal of Environmental Research and Public Health, 11 (5), 4745–4767. https://doi.org/10.3390/ijerph110504745
- Hidalgo-Cantabrana, C., Delgado, S., Ruiz, L., Ruas-Madiedo, P., Sánchez, Hidalgo-Cantabrana, C., Delgado, S. et al. (2018). Bifidobacteria and Their Health-Promoting Effects. Bugs as Drugs, 73–98. https://doi.org/10.1128/9781555819705.ch3
- Arboleya, S., Watkins, C., Stanton, C., Ross, R. P. (2016). Gut Bifidobacteria Populations in Human Health and Aging. Frontiers in Microbiology, 7. https://doi.org/10.3389/fmicb.2016.01204
- Tang, X., Tian, Q., Cheng, X., Li, N., Mao, X. (2013). Bifidobacterial growth–promoting effect of yak milk κ‐casein hydrolysates produced with different proteases. International Journal of Food Science & Technology, 48 (8), 1682–1687. https://doi.org/10.1111/ijfs.12138
- Reuter, G. (2001). The Lactobacillus and Bifidobacterium Microflora of the Human Intestine: Composition and Succession. Current issues in intestinal microbiology, 2 (2), 43–53. Available at: https://pubmed.ncbi.nlm.nih.gov/11721280/
- Fratianni, F., Pepe, S., Cardinale, F., Granese, T., Cozzolino, A., Coppola, R., Nazzaro, F. (2014). Eruca sativa Might Influence the Growth, Survival under Simulated Gastrointestinal Conditions and Some Biological Features of Lactobacillus acidophilus, Lactobacillus plantarum and Lactobacillus rhamnosus Strains. International Journal of Molecular Sciences, 15 (10), 17790–17805. https://doi.org/10.3390/ijms151017790
- Kim, H. S., Jeong, S. G., Ham, J. S., Chae, H. S., Lee, J. M., Ahn, C. N. (2006). Antioxidative and Probiotic Properties of Lactobacillus gasseri NLRI-312 Isolated from Korean Infant Feces. Asian-Australasian Journal of Animal Sciences, 19 (9), 1335–1341. https://doi.org/10.5713/ajas.2006.1335
- Bibi, A., Xiong, Y., Rajoka, M. S. R., Mehwish, H. M., Radicetti, E., Umair, M. et al. (2021). Recent Advances in the Production of Exopolysaccharide (EPS) from Lactobacillus spp. and Its Application in the Food Industry: A Review. Sustainability, 13 (22), 12429. https://doi.org/10.3390/su132212429
- Hsu, C. A., Yu, R. C., Chou, C. C. (2005). Production of β-galactosidase by Bifidobacteria as influenced by various culture conditions. International Journal of Food Microbiology, 104 (2), 197–206. https://doi.org/10.1016/j.ijfoodmicro.2005.02.010
- Kolev, P., Rocha-Mendoza, D., Ruiz-Ramírez, S., Ortega-Anaya, J., Jiménez-Flores, R., García-Cano, I. (2022). Screening and characterization of β-galactosidase activity in lactic acid bacteria for the valorization of acid whey. JDS Communications, 3 (1), 1–6. https://doi.org/10.3168/jdsc.2021-0145
- Kumar, M., Nagpal, R., Kumar, R., Hemalatha, R., Verma, V., Kumar, A. et al. (2012). Cholesterol-Lowering Probiotics as Potential Biotherapeutics for Metabolic Diseases. Experimental Diabetes Research, 2012, 1–14. https://doi.org/10.1155/2012/902917
- Chen, J., Chen, X., Ho, C. L. (2021). Recent Development of Probiotic Bifidobacteria for Treating Human Diseases. Frontiers in Bioengineering and Biotechnology, 9. https://doi.org/10.3389/fbioe.2021.770248
- Pereira, L., Souza, C., Behrens, J., Saad, S. (2010). Lactobacillus acidophilus and Bifidobacterium sp. In co-culture improve sensory acceptance of potentially probiotic petit-suisse cheese. Acta Alimentaria, 39 (3), 265–276. https://doi.org/10.1556/aalim.39.2010.3.3
- Szajnar, K., Znamirowska, A., Kuźniar, P. (2020). Sensory and textural properties of fermented milk with viability of Lactobacillus rhamnosus and Bifidobacterium animalis ssp. lactis Bb-12 and increased calcium concentration. International Journal of Food Properties, 23 (1), 582–598. https://doi.org/10.1080/10942912.2020.1748050
- Ndhlala, A. R., Kavaz Yüksel, A., Yüksel, M. (2022). Nutritional Supplementation of Yogurt with Jerusalem Artichoke Tubers: Organic Acid Profiles and Quality Parameters. Plants, 11 (22), 3086. https://doi.org/10.3390/plants11223086
- Bennato, F., Ianni, A., Innosa, D., Martino, C., Grotta, L., Pomilio, F. et al. (2019). Influence of Licorice Root Feeding on Chemical-Nutritional Quality of Cow Milk and Stracciata Cheese, an Italian Traditional Fresh Dairy Product. Animals, 9 (12), 1153. https://doi.org/10.3390/ani9121153
- Jia, R., Hu, X., Zhang, Y. (2023). Development of Licorice Flavored Fermented Milk. China Dairy, 4, 101–105. https://doi.org/10.12377/1671-4393.23.04.17
- Glagoleva, L. E., Zatsepilina, N. P., Nesterenko, I. P., Pevneva, D. M. (2021). About the use of plant-based complex of alfalfa in production of dairy products. IOP Conference Series: Earth and Environmental Science, 848 (1), 012049. https://doi.org/10.1088/1755-1315/848/1/012049
- Li, N., Wang, Z., Sun, C., Zhao, S., Jiang, L., Zhao, C. et al. (2019). Burdock polysaccharides enhanced the quality and antioxidative activity of fermented milk. Food and Fermentation Industries, 45 (10), 97–103. Available at: http://sf1970.cnif.cn/CN/10.13995/j.cnki.11-1802/ts.019325
- Prosekov, A. Y., Dyshlyuk, L. S., Milent`eva, I. S., Pavsky, V. A., Ivanova, S. A., Garmashov, S. Y. (2018). Study of the biofunctional properties of cedar pine oil with the use of testing cultures. Foods and Raw Materials, 6 (1), 136–143. https://doi.org/10.21603/2308-4057-2018-1-136-143
- Khamagaeva, I. S., Zambalova, N. A., Tsyzhipova, A. V., Bubeev, A. T. (2020). The development of a biologically active additive to reduce the blood cholesterol level. E3S Web of Conferences, 161, 01093. https://doi.org/10.1051/e3sconf/202016101093
- Donno, D., Mellano, M., De Biaggi, M., Riondato, I., Rakotoniaina, E., Beccaro, G. (2018). New Findings in Prunus padus L. Fruits as a Source of Natural Compounds: Characterization of Metabolite Profiles and Preliminary Evaluation of Antioxidant Activity. Molecules, 23 (4), 725. https://doi.org/10.3390/molecules23040725
- Güney, D., Güngörmüşler, M. (2020). Development and Comparative Evaluation of a Novel Fermented Juice Mixture with Probiotic Strains of Lactic Acid Bacteria and Bifidobacteria. Probiotics and Antimicrobial Proteins, 13 (2), 495–505. https://doi.org/10.1007/s12602-020-09710-2
- Servili, M., Rizzello, C. G., Taticchi, A., Esposto, S., Urbani, S., Mazzacane, F. et al. (2011). Functional milk beverage fortified with phenolic compounds extracted from olive vegetation water, and fermented with functional lactic acid bacteria. International Journal of Food Microbiology, 147 (1), 45–52. https://doi.org/10.1016/j.ijfoodmicro.2011.03.006
- Amirdivani, S., Baba, A. S. H. (2014). Green tea yogurt: major phenolic compounds and microbial growth. Journal of Food Science and Technology, 52 (7), 4652–4660. https://doi.org/10.1007/s13197-014-1670-6
- Mediza Romero, M. L., von Staszewski, M., Martínez, M. J. (2021). The effect of green tea polyphenols addition on the physicochemical, microbiological and bioactive characteristics of yogurt. British Food Journal, 123 (7), 2380–2397. https://doi.org/10.1108/bfj-07-2020-0648
- Rahmani, F., Gandomi, H., Noori, N., Faraki, A., Farzaneh, M. (2021). Microbial, physiochemical and functional properties of probiotic yogurt containing Lactobacillus acidophilus and Bifidobacterium bifidum enriched by green tea aqueous extract. Food Science & Nutrition, 9 (10), 5536–5545. https://doi.org/10.1002/fsn3.2512
- Shori, A. B., Muniandy, P., Baba, A. S. (2021). Changes in Phenolic Compounds Profiles in Tea Extracts and the Composition of these Phenolic Compounds in Yogurt. Recent Patents on Food, Nutrition & Agriculture, 12 (1), 36–44. https://doi.org/10.2174/2212798411999201123205022
- Larasati, B. A., Panunggal, B., Afifah, D. N., Anjani, G., Rustanti, N. (2018). Total lactic acid bacteria, antioxidant activity, and acceptance of synbiotic yoghurt with red ginger extract (Zingiberofficinale var. rubrum). IOP Conference Series: Earth and Environmental Science, 116, 012037. https://doi.org/10.1088/1755-1315/116/1/012037
- Telichowska, A., Kobus-Cisowska, J., Szulc, P. (2020). Phytopharmacological Possibilities of Bird Cherry Prunus padus L. and Prunus serotina L. Species and Their Bioactive Phytochemicals. Nutrients, 12 (7), 1966. https://doi.org/10.3390/nu12071966
- Telichowska, A., Kobus-Cisowska, J., Ligaj, M., Stuper-Szablewska, K., Szymanowska, D., Tichoniuk, M., Szulc, P. (2020). Polyphenol content and antioxidant activities of Prunus padus L. and Prunus serotina L. leaves: Electrochemical and spectrophotometric approach and their antimicrobial properties. Open Chemistry, 18 (1), 1125–1135. https://doi.org/10.1515/chem-2020-0121
- Parfenov, A. A., Vyshtakalyuk, A. B., Sysoeva, M. A., Sysoeva, E. V., Latipova, A. D., Gumarova, L. F., Zobov, V. V. (2019). Hepatoprotective Effect of Inonotus obliquus Melanins: In Vitro and In Vivo Studies. BioNanoScience, 9 (2), 528–538. https://doi.org/10.1007/s12668-019-0595-y
- GOST 8.639-2014. State system for ensuring the uniformity of measurements. Measuring electrodes for determination of oxidation–reduction potential. Verification procedure (2014). Federal Agency on Technical Regulating and Metrology.
- GOST 55312-2012. Propolis. Method for determination of flavonoid compositions (2012). Federal Agency on Technical Regulating and Metrology.
- GOST 33491-2015. Product fermented-milk, enriched bifidobacteriae bifidum. Specifications (2015). Federal Agency on Technical Regulating and Metrology.
- Methodical instructions MUK 4.2.577-96. Methods of microbiological control of children's and therapeutic food products and their components.
- Tastemirova, U., Mukhtarkhanova, R., Alimardanova, M., Alibekov, R., Shingisov, A. (2022). Impact of vacuum freeze-drying on the reconstituted camel milk composition. Food Science and Technology, 42. https://doi.org/10.1590/fst.61722
- GOST 54340-2011. Fermented dairy and dairy compound products. General specifications (2011). Federal Agency on Technical Regulating and Metrology.
- Patel, S., Goyal, A. (2012). The current trends and future perspectives of prebiotics research: a review. 3 Biotech, 2 (2), 115–125. https://doi.org/10.1007/s13205-012-0044-x
- Al-Hindi, R. R., Abd El Ghani, S. (2020). Production of Functional Fermented Milk Beverages Supplemented with Pomegranate Peel Extract and Probiotic Lactic Acid Bacteria. Journal of Food Quality, 2020, 1–9. https://doi.org/10.1155/2020/4710273
- Linares, D. M., Gómez, C., Renes, E., Fresno, J. M., Tornadijo, M. E., Ross, R. P., Stanton, C. (2017). Lactic Acid Bacteria and Bifidobacteria with Potential to Design Natural Biofunctional Health-Promoting Dairy Foods. Frontiers in Microbiology, 8. https://doi.org/10.3389/fmicb.2017.00846
- Melini, F., Melini, V., Luziatelli, F., Ficca, A. G., Ruzzi, M. (2019). Health-Promoting Components in Fermented Foods: An Up-to-Date Systematic Review. Nutrients, 11 (5), 1189. https://doi.org/10.3390/nu11051189
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2024 Aidana Utebaeva, Eleonora Gabrilyants, Zhansaya Abish, Viktoriia Yevlash

This work is licensed under a Creative Commons Attribution 4.0 International License.
The consolidation and conditions for the transfer of copyright (identification of authorship) is carried out in the License Agreement. In particular, the authors reserve the right to the authorship of their manuscript and transfer the first publication of this work to the journal under the terms of the Creative Commons CC BY license. At the same time, they have the right to conclude on their own additional agreements concerning the non-exclusive distribution of the work in the form in which it was published by this journal, but provided that the link to the first publication of the article in this journal is preserved.
A license agreement is a document in which the author warrants that he/she owns all copyright for the work (manuscript, article, etc.).
The authors, signing the License Agreement with TECHNOLOGY CENTER PC, have all rights to the further use of their work, provided that they link to our edition in which the work was published.
According to the terms of the License Agreement, the Publisher TECHNOLOGY CENTER PC does not take away your copyrights and receives permission from the authors to use and dissemination of the publication through the world's scientific resources (own electronic resources, scientometric databases, repositories, libraries, etc.).
In the absence of a signed License Agreement or in the absence of this agreement of identifiers allowing to identify the identity of the author, the editors have no right to work with the manuscript.
It is important to remember that there is another type of agreement between authors and publishers – when copyright is transferred from the authors to the publisher. In this case, the authors lose ownership of their work and may not use it in any way.








