Identification of the oxidation and hydrolysis products content influence on the rapeseed oil oxidation induction period
DOI:
https://doi.org/10.15587/1729-4061.2024.308907Keywords:
refined rapeseed oil, primary oxidation products, free fatty acids, accelerated oxidationAbstract
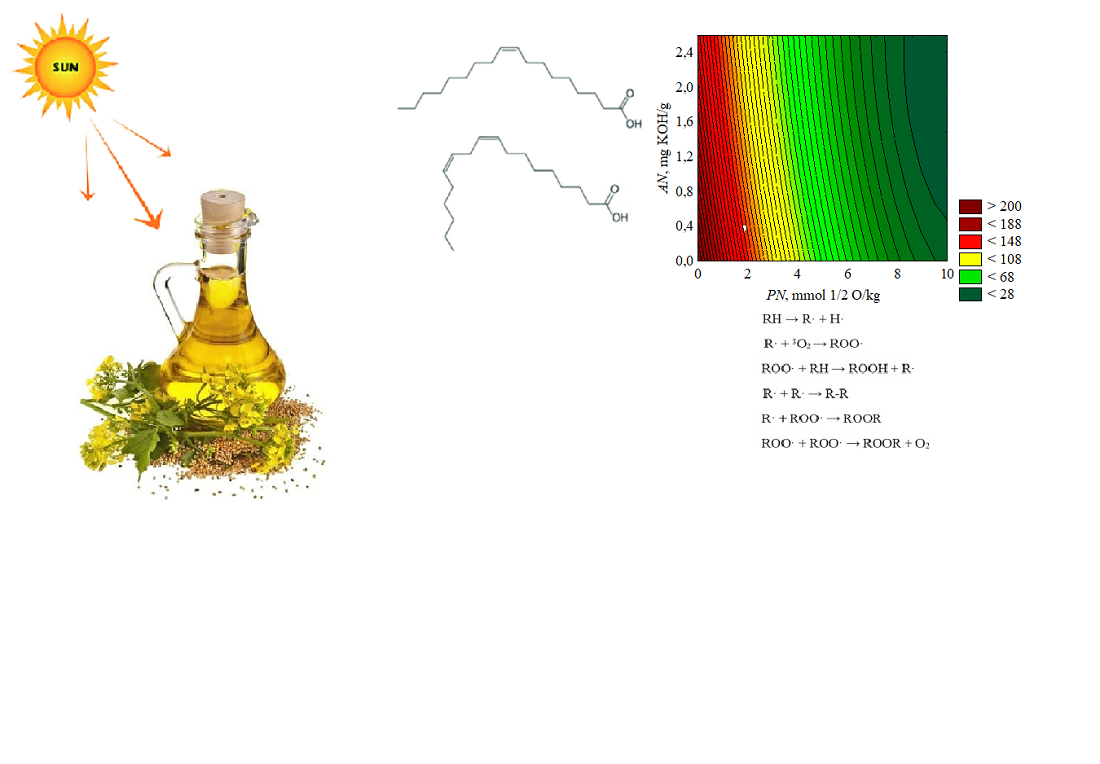
The object of the study is the dependence of the induction period of rapeseed oil on the content of oxidation and hydrolysis products. A feature of the work is determining the approximation dependence of the induction period of accelerated oxidation of refined rapeseed oil on the content of primary oxidation products and free fatty acids. This is useful when predicting the shelf life of refined rapeseed oil. It was determined that both factors negatively affect the oxidation stability of refined rapeseed oil. An increase in the peroxide value decreases the induction period of model oil samples by 32.8512 units for each additional mmol ½ O/kg. In turn, increasing the acid value of oil samples reduces the induction period by 19.8424 units for each additional mg KOH/g. Different oxidation dynamics of model samples of refined rapeseed oil with tocopherol were revealed, depending on the concentration of primary oxidation and hydrolysis products. The obtained data are explained by the fact that the primary lipid oxidation products are unstable and quickly decompose to form free radicals. These radicals initiate further lipid oxidation, resulting in reduced oil quality. In addition, free fatty acids are more reactive than triglycerides and are more easily oxidized. A feature of the obtained results is the possibility of modeling processes that affect the oxidation stability of refined rapeseed oil. From a practical point of view, the research results allow initiating measures to maintain the safety of oil-containing food products based on refined rapeseed oil. An applied aspect of using the scientific results is the possibility of rationalizing the storage conditions of refined rapeseed oil to maximize its shelf life and increase competitiveness
References
- Boldyryev, S., Khussanov, A., Gorbunov, K., Gorbunova, O. (2019). Sustainability improvement of Kazakh chemical industry via process integration: A case study of calcium chloride production. Chemical Engineering Transactions, 76, 1231–1236. https://doi.org/10.3303/CET1976206
- Ved, V., Ponomarenko, H., Ponomarenko, Y., Gorbunov, K. (2021). A Modified Scheffe’s Simplex Lattice Design Method in Development of Ceramic Carriers for Catalytic Neutralizers of Gas Emissions. Chemistry Journal of Moldova, 16 (1), 79–87. https://doi.org/10.19261/cjm.2021.779
- Abad, A., Shahidi, F. (2021). Fatty acid, triacylglycerol and minor component profiles affect oxidative stability of camelina and sophia seed oils. Food Bioscience, 40, 100849. https://doi.org/10.1016/j.fbio.2020.100849
- Ali, M. A., Nargis, A., Othman, N. H., Noor, A. F., Sadik, G., Hossen, J. (2017). Oxidation stability and compositional characteristics of oils from microwave roasted pumpkin seeds during thermal oxidation. International Journal of Food Properties, 20 (11), 2569–2580. https://doi.org/10.1080/10942912.2016.1244544
- Belinska, A., Bliznjuk, O., Masalitina, N., Bielykh, I., Zviahintseva, O., Gontar, T. et al. (2023). Development of biotechnologically transesterified three-component fat systems stable to oxidation. Eastern-European Journal of Enterprise Technologies, 5 (6 (125)), 21–28. https://doi.org/10.15587/1729-4061.2023.287326
- Wang, D., Xiao, H., Lyu, X., Chen, H., Wei, F. (2023). Lipid oxidation in food science and nutritional health: A comprehensive review. Oil Crop Science, 8 (1), 35–44. https://doi.org/10.1016/j.ocsci.2023.02.002
- Yuan, L., Xu, Z., Tan, C.-P., Liu, Y., Xu, Y.-J. (2021). Biohazard and dynamic features of different polar compounds in vegetable oil during thermal oxidation. LWT, 146, 111450. https://doi.org/10.1016/j.lwt.2021.111450
- Ma, G., Wang, Y., Li, Y., Zhang, L., Gao, Y., Li, Q., Yu, X. (2023). Antioxidant properties of lipid concomitants in edible oils: A review. Food Chemistry, 422, 136219. https://doi.org/10.1016/j.foodchem.2023.136219
- Maszewska, M., Florowska, A., Dłużewska, E., Wroniak, M., Marciniak-Lukasiak, K., Żbikowska, A. (2018). Oxidative Stability of Selected Edible Oils. Molecules, 23 (7), 1746. https://doi.org/10.3390/molecules23071746
- Jiang, L., Wu, W., Wu, S., Wu, J., Zhang, Y., Liao, L. (2024). Effect of different pretreatment techniques on quality characteristics, chemical composition, antioxidant capacity and flavor of cold-pressed rapeseed oil. LWT, 201, 116157. https://doi.org/10.1016/j.lwt.2024.116157
- Li, Z., Wang, W., Liu, X., Qi, S., Lan, D., Wang, Y. (2023). Effect of different degumming processes on the retention of bioactive components, acylglycerol and phospholipid composition of rapeseed oil. Process Biochemistry, 133, 190–199. https://doi.org/10.1016/j.procbio.2023.08.019
- Arabsorkhi, B., Pourabdollah, E., Mashadi, M. (2023). Investigating the effect of replacing the antioxidants Ascorbyl palmitate and tocopherol instead of TBHQ on the shelf life of sunflower oil using temperature accelerated method. Food Chemistry Advances, 2, 100246. https://doi.org/10.1016/j.focha.2023.100246
- Ayu, D. F., Andarwulan, N., Hariyadi, P., Purnomo, E. H. (2016). Effect of tocopherols, tocotrienols, β-carotene, and chlorophyll on the photo-oxidative stability of red palm oil. Food Science and Biotechnology, 25 (2), 401–407. https://doi.org/10.1007/s10068-016-0055-1
- Gulsen, K., Cakmak-Arslan, G. (2023). Evaluation of the antioxidant effect of propolis on thermal oxidation of sunflower oil using ATR-MIR spectroscopy. Chemical Papers, 77 (10), 5733–5750. https://doi.org/10.1007/s11696-023-02893-2
- Lehnert, S., Dubinina, A., Deynichenko, G., Khomenko, O., Haponceva, O., Antonyuk, I. et al. (2018). The study of influence of natural antioxidants on quality of peanut and linseed oil blends during their storage. Eastern-European Journal of Enterprise Technologies, 3 (11 (93)), 44–50. https://doi.org/10.15587/1729-4061.2018.133433
- Petik, P., Stankevych, S., Zabrodina, I., Zhulinska, O., Mezentseva, I., Haliasnyi, I. et al. (2023). Determination of fat-soluble dyes influence on the oxidation induction period of their oil solutions. Eastern-European Journal of Enterprise Technologies, 3 (6 (123)), 13–21. https://doi.org/10.15587/1729-4061.2023.279619
- Almeida, E. S., Carmona, P. O., Mendonça, S., Dias, A. C. B., Castellón, E. R., Cecilia, J. A. et al. (2024). The role of carotenes in preventing oxidation during palm oil processing: Adsorption studies. Industrial Crops and Products, 216, 118691. https://doi.org/10.1016/j.indcrop.2024.118691
- Safarzadeh Markhali, F., Teixeira, J. A. (2024). Stability of target polyphenols of leaf-added virgin olive oil under different storage conditions over time. Sustainable Food Technology, 2 (3), 780–789. https://doi.org/10.1039/d4fb00068d
- Ali, M. A., Chew, S. C., Majid, F. A. A. (2022). Contribution of endogenous minor components in the oxidative stability of rice bran oil. Journal of Food Measurement and Characterization, 17 (1), 187–210. https://doi.org/10.1007/s11694-022-01602-z
- Maghsoudlou, E., Raftani Amiri, Z., Esmaeilzadeh kenari, R. (2023). Determination and correlation analysis of phytochemical compounds, antioxidant activity, and oxidative stability of different edible oils. Journal of Food Measurement and Characterization, 18 (1), 714–726. https://doi.org/10.1007/s11694-023-02241-8
- de Carvalho, A. G. A., Silva, L. de O., Monteiro, M., Perrone, D., Castelo-Branco, V. N., Torres, A. G. (2024). Jussara palm tree (Euterpe edulis M.) açai-berry oil performance on auto- and photo-oxidation: Minor components’ stability and evolution of volatile compounds. Food Chemistry Advances, 4, 100618. https://doi.org/10.1016/j.focha.2024.100618
- Oliya ripakova Kujawski. Available at: https://oleina.ua/kujawski/product/ripakova-kujawski
- Setting Up a DSC Oxygen Induction Time Procedure. Available at: https://folk.ntnu.no/deng/fra_nt/other%20stuff/DSC_manuals/QDSC/Setting_Up_a_DSC_Oxygen_Induction_Time_Procedure.htm
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2024 Serhii Stankevych, Kostiantyn Gorbunov, Inna Zabrodina, Mykola Popov, Viktoriia Kalyna, Tetiana Novozhylova, Tetiana Falalieieva, Tetiana Ovsiannikova, Maryna Ponomarova, Andrii Zolotarov

This work is licensed under a Creative Commons Attribution 4.0 International License.
The consolidation and conditions for the transfer of copyright (identification of authorship) is carried out in the License Agreement. In particular, the authors reserve the right to the authorship of their manuscript and transfer the first publication of this work to the journal under the terms of the Creative Commons CC BY license. At the same time, they have the right to conclude on their own additional agreements concerning the non-exclusive distribution of the work in the form in which it was published by this journal, but provided that the link to the first publication of the article in this journal is preserved.
A license agreement is a document in which the author warrants that he/she owns all copyright for the work (manuscript, article, etc.).
The authors, signing the License Agreement with TECHNOLOGY CENTER PC, have all rights to the further use of their work, provided that they link to our edition in which the work was published.
According to the terms of the License Agreement, the Publisher TECHNOLOGY CENTER PC does not take away your copyrights and receives permission from the authors to use and dissemination of the publication through the world's scientific resources (own electronic resources, scientometric databases, repositories, libraries, etc.).
In the absence of a signed License Agreement or in the absence of this agreement of identifiers allowing to identify the identity of the author, the editors have no right to work with the manuscript.
It is important to remember that there is another type of agreement between authors and publishers – when copyright is transferred from the authors to the publisher. In this case, the authors lose ownership of their work and may not use it in any way.








