Combination of electrophoretic systems for determining of the fractional composition of proteins in lactoferrin preparations
DOI:
https://doi.org/10.15587/1729-4061.2025.338604Keywords:
lactoferrin preparations, gel filtration, electrophoresis, protein fractions, whey proteins, caseinsAbstract
The object of this study is three lactoferrin (LF) preparations used as bioactive supplements. The problem of determining the fractional composition of proteins in lactoferrin preparations was solved.
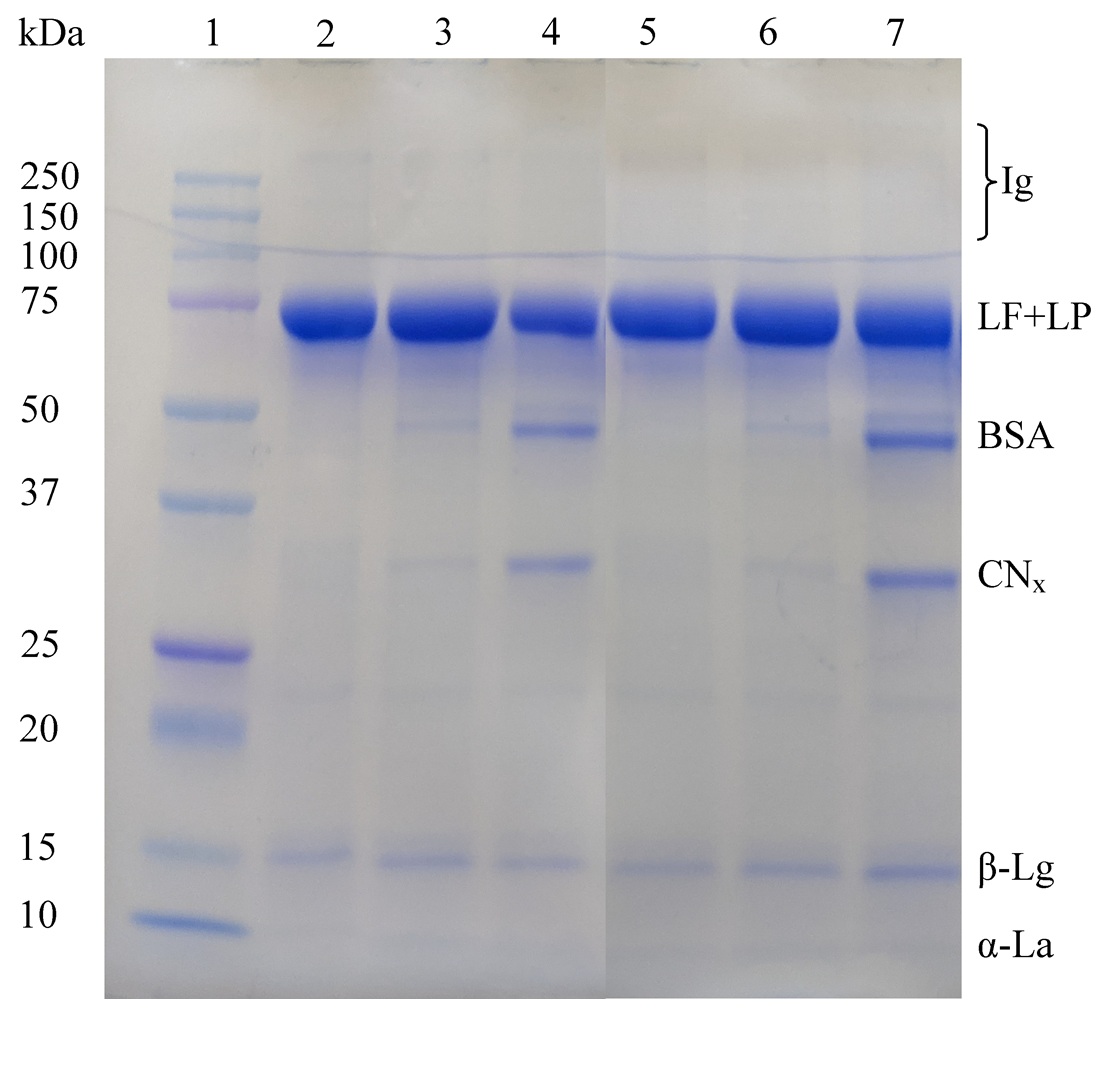
The molecular weights of proteins in LF preparations have been determined by gel filtration on Sephadex G-25 and G-100. It was established that all three preparations contain proteins which molecular weights, including LF, are in the range from 5000 to 100000 Da. Low-molecular-weight (about 1000 Da) peptides were found in one preparation, which constitute 9 ± 3% of all protein impurities. Four different electrophoretic systems in polyacrylamide gel (PAG) were used to identify proteins in LF preparations. It was found that in order to detect whey protein fractions in preparations it is advisable to combine electrophoretic systems under native conditions with a disc electrophoresis system in the presence of sodium dodecylsulfate. Casein fractions can be detected by combining electrophoresis in the presence of urea and disc electrophoresis with SDS. Quantitative analysis of the relative content of impurity proteins was performed by the densitometry of PAG plates after disc electrophoresis in the presence of SDS. In the studied preparations (LF1, LF2, LF3), in addition to LF, β-lactoglobulin (β-Lg), blood serum albumin (BSA), and αS1-casein (αS1-CN) were detected. The relative content of these fractions from all proteins in the preparations is as follows: in LF1 – β-Lg (3 ± 0.4%), αS1-CN (< 1%), BSA (< 1%); in LF2 – β-Lg (3 ± 0.3%), αS1-CN (1 ± 0.2%), BSA (1 ± 0.3%) and in LF3 – β-Lg (3 ± 0.4%), αS1-CN (1 ± 0.2%), BSA (5 ± 0.6%). All the studied LF preparations differ in the content and ratio of protein fractions, which may indicate the need to analyze the protein composition of each batch of the preparation
References
- Kell, D. B., Heyden, E. L., Pretorius, E. (2020). The Biology of Lactoferrin, an Iron-Binding Protein That Can Help Defend Against Viruses and Bacteria. Frontiers in Immunology, 11. https://doi.org/10.3389/fimmu.2020.01221
- Cutone, A., Rosa, L., Ianiro, G., Lepanto, M. S., Bonaccorsi di Patti, M. C., Valenti, P., Musci, G. (2020). Lactoferrin’s Anti-Cancer Properties: Safety, Selectivity, and Wide Range of Action. Biomolecules, 10 (3), 456. https://doi.org/10.3390/biom10030456
- Min, Q.-Q., Qin, L.-Q., Sun, Z.-Z., Zuo, W.-T., Zhao, L., Xu, J.-Y. (2018). Effects of Metformin Combined with Lactoferrin on Lipid Accumulation and Metabolism in Mice Fed with High-Fat Diet. Nutrients, 10 (11), 1628. https://doi.org/10.3390/nu10111628
- Superti, F. (2020). Lactoferrin from Bovine Milk: A Protective Companion for Life. Nutrients, 12 (9), 2562. https://doi.org/10.3390/nu12092562
- Duran, A., Kahve, H. I. (2017). The use of lactoferrin in food industry. Academic Journal of Science, 07 (02), 89–94. Available at: https://universitypublications.net/ajs/0702/html/DE6C406.xml
- Slyvka, I., Tsisaryk, O., Musii, L., Kushnir, I., Koziorowski, M., Koziorowska, A. (2022). Identification and Investigation of properties of strains Enterococcus spp. Isolated from artisanal Carpathian cheese. Biocatalysis and Agricultural Biotechnology, 39, 102259. https://doi.org/10.1016/j.bcab.2021.102259
- Tsizh, B., Skulska, I., Tsisaryk, O., Slyvka, N., Musiy, L., Slyvka, I. (2024). Characteristics of the course of proteolytic processes in brynza with reduced common salt content. Zywnosc Nauka Technologia Jakosc/Food Science Technology Quality, 31 (4), 23–44. https://doi.org/10.15193/zntj/2024/141/519
- Fox, P. F., Uniacke-Lowe, T., McSweeney, P. L. H., O’Mahony, J. A. (2015). Dairy Chemistry and Biochemistry. Springer International Publishing. https://doi.org/10.1007/978-3-319-14892-2
- Krolitzki, E., Schwaminger, S. P., Pagel, M., Ostertag, F., Hinrichs, J., Berensmeier, S. (2022). Current practices with commercial scale bovine lactoferrin production and alternative approaches. International Dairy Journal, 126, 105263. https://doi.org/10.1016/j.idairyj.2021.105263
- Wakabayashi, H., Yamauchi, K., Abe, F. (2018). Quality control of commercial bovine lactoferrin. BioMetals, 31 (3), 313–319. https://doi.org/10.1007/s10534-018-0098-2
- Yukalo, V. H. (2021). Biolohichna aktyvnist proteiniv i peptydiv moloka. Ternopil: Vyd-vo TNTU imeni Ivana Puliuia, 372. Available at: https://elartu.tntu.edu.ua/handle/lib/36801
- Laemmli, U. K. (1970). Cleavage of Structural Proteins during the Assembly of the Head of Bacteriophage T4. Nature, 227 (5259), 680–685. https://doi.org/10.1038/227680a0
- Perraudin, J.-P., Valck, L. D. (2020). Lactoferrin Production from Bovine Milk or Cheese Whey. Journal of Engineering and Applied Sciences Technology, 2 (1), 1–9. https://doi.org/10.47363/jeast/2020(2)103
- Deeth, H. C., Bansal, N. (Eds.) (2019). Whey Proteins: From Milk to Medicine. Academic Press. https://doi.org/10.1016/c2016-0-02581-0
- Sharma, N., Sharma, R., Rajput, Y. S., Mann, B., Singh, R., Gandhi, K. (2021). Separation methods for milk proteins on polyacrylamide gel electrophoresis: Critical analysis and options for better resolution. International Dairy Journal, 114, 104920. https://doi.org/10.1016/j.idairyj.2020.104920
- Yukalo, V., Datsyshyn, K., Krupa, O., Storozh, L. (2024). Adaptation of Stadier’s apparatus for electrophoresis of main milk proteins. Eastern-European Journal of Enterprise Technologies, 1 (11 (127)), 73–80. https://doi.org/10.15587/1729-4061.2024.296753
- Yukalo, V., Datsyshyn, K., Storozh, L. (2019). Electrophoretic system for express analysis of whey protein fractions. Eastern-European Journal of Enterprise Technologies, 2 (11 (98)), 37–44. https://doi.org/10.15587/1729-4061.2019.160186
- Tarapata, J., Maciejczyk, M., Zulewska, J. (2022). Microfiltration of buttermilk: Partitioning of proteins and modelling using a resistance-in-series model. International Dairy Journal, 134, 105445. https://doi.org/10.1016/j.idairyj.2022.105445
- Kukhtyn, M., Kremenchuk, I., Horiuk, Y., Salata, V., Kochetova, H., Kladnytska, L. et al. (2025). Development and evaluation of technology for preserving hard cheese with staphylococcal bacteriophage. Scifood, 19, 208–223. https://doi.org/10.5219/scifood.16
- Kukhtyn, M., Arutiunian, D., Pokotylo, O., Kravcheniuk, K., Salata, V., Horiuk, Y. et al. (2024). Microbiological characteristics of hard cheese with flax seeds. Potravinarstvo Slovak Journal of Food Sciences, 18, 281–296. https://doi.org/10.5219/1956
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2025 Volodymyr Yukalo, Kateryna Datsyshyn, Olha Krupa, Liudmyla Storozh

This work is licensed under a Creative Commons Attribution 4.0 International License.
The consolidation and conditions for the transfer of copyright (identification of authorship) is carried out in the License Agreement. In particular, the authors reserve the right to the authorship of their manuscript and transfer the first publication of this work to the journal under the terms of the Creative Commons CC BY license. At the same time, they have the right to conclude on their own additional agreements concerning the non-exclusive distribution of the work in the form in which it was published by this journal, but provided that the link to the first publication of the article in this journal is preserved.
A license agreement is a document in which the author warrants that he/she owns all copyright for the work (manuscript, article, etc.).
The authors, signing the License Agreement with TECHNOLOGY CENTER PC, have all rights to the further use of their work, provided that they link to our edition in which the work was published.
According to the terms of the License Agreement, the Publisher TECHNOLOGY CENTER PC does not take away your copyrights and receives permission from the authors to use and dissemination of the publication through the world's scientific resources (own electronic resources, scientometric databases, repositories, libraries, etc.).
In the absence of a signed License Agreement or in the absence of this agreement of identifiers allowing to identify the identity of the author, the editors have no right to work with the manuscript.
It is important to remember that there is another type of agreement between authors and publishers – when copyright is transferred from the authors to the publisher. In this case, the authors lose ownership of their work and may not use it in any way.








