Unraveling the study of liquid smoke from rice husks as a green corrosion inhibitor in mild steel under 1 M HCl
DOI:
https://doi.org/10.15587/1729-4061.2022.265086Keywords:
liquid smoke, green corrosion inhibitor, rice husks ash, chemisorptionsAbstract
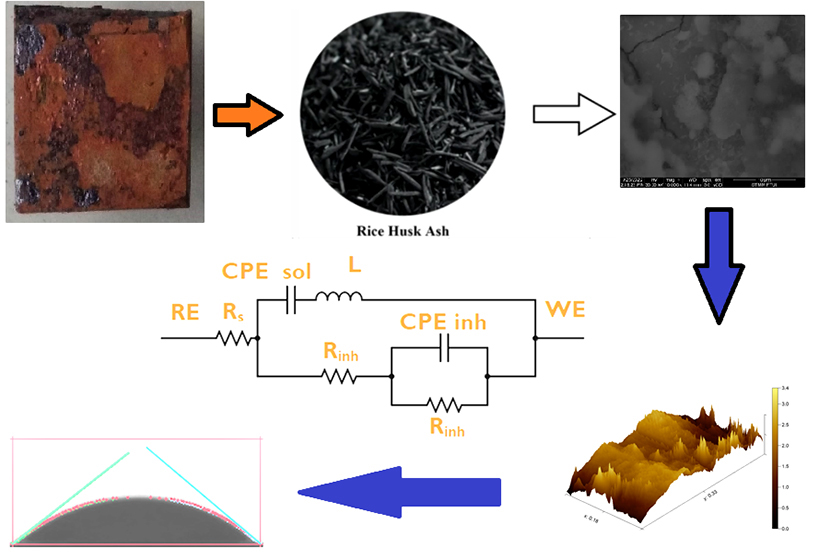
The work provides the more comprehensive development of Liquid Smoke from Rice Husks Ash (RHA). Notably, the study focuses on the interaction between the primary molecules of inhibitor and mild steel, including thermodynamic calculation and surface treatment upon addition of inhibitor. The electrochemical impedance spectroscopy (EIS) and potentiodynamic polarization (PP) characterization were utilized to evaluate the anticorrosion of RHA. The Raman Spectroscopy pre and post-addition of RHA’s inhibitor were used to compare the adsorbed functional group of inhibitors. Moreover, the thermodynamic calculation of the inhibitor’s adsorption determines the types of adsorption of the inhibitor. As a result of the adsorption process, the Scanning Electronic Microscope-Energy Dispersive X-Ray (SEM-EDX) aided by The Atomic Force Microscopy (AFM) and Contact Angle Test was implemented to unveil the surface treatment and the change of elemental composition after the addition of an 80 ppm inhibitor. The PP and EIS results show a significant depression of the current density at ‒2.75 μA·cm2 in 80 ppm solution with the highest inhibition efficiency of 99.82 %. The superior inhibition correlates to the adsorption of Si–OH, C–C, C–O–C, >C=O, complex structure, and –OH at wavenumber 458, 662, 1095, 1780, and 3530 cm-1. The LS shows a significant surface area of protection of 0.9982 and high adsorption constant (Kads) at 11.648. The calculated ΔGads of ‒6.59 kJ/mol unveils the chemisorption in nature. At the same time, a combination of 20 and 80 ppm solution is predicted adsorbed horizontally to reduce the contact between the solution and substrate, as shown in SEM and AFM results. It also increases the contact angle and their corresponding hydrophobicity
Supporting Agency
- The author gratefully thanks the Ministry of Research and Technology/National Research and Innovation Agency for the financial support of contract number NKB-1008/UN2.RST/HKP.05.00/2022.
References
- Guillal, A., Ben Seghier, M. E. A., Nourddine, A., Correia, J. A. F. O., Bt Mustaffa, Z., Trung, N.-T. (2020). Probabilistic investigation on the reliability assessment of mid- and high-strength pipelines under corrosion and fracture conditions. Engineering Failure Analysis, 118, 104891. doi: https://doi.org/10.1016/j.engfailanal.2020.104891
- Simmons, M. R. (2008). Report of offshore technology conference (OTC) presentation. Houston, TX: NACE International Oil and Gas Production.
- Gupta, N. K., Verma, C., Salghi, R., Lgaz, H., Mukherjee, A. K., Quraishi, M. A. (2017). New phosphonate based corrosion inhibitors for mild steel in hydrochloric acid useful for industrial pickling processes: experimental and theoretical approach. New Journal of Chemistry, 41 (21), 13114–13129. doi: https://doi.org/10.1039/c7nj01431g
- Aditiyawarman, T., Kaban, A. P. S., Soedarsono, J. W. (2022). A Recent Review of Risk-Based Inspection Development to Support Service Excellence in the Oil and Gas Industry: An Artificial Intelligence Perspective. ASCE-ASME J Risk and Uncert in Engrg Sys Part B Mech Engrg, 9 (1). doi: https://doi.org/10.1115/1.4054558
- Aditiyawarman, T., Soedarsono, J. W., Kaban, A. P. S., Riastuti, R., Rahmadani, H. (2022). The Study of Artificial Intelligent in Risk-Based Inspection Assessment and Screening: A Study Case of Inline Inspection. ASCE-ASME Journal of Risk and Uncertainty in Engineering Systems, Part B: Mechanical Engineering, 9 (1). doi: https://doi.org/10.1115/1.4054969
- Pumps, S. (2010). Materials and Corrosion. Centrifugal Pump Handbook, 227–250. doi: https://doi.org/10.1016/b978-0-7506-8612-9.00008-5
- Wasim, M., Djukic, M. B. (2021). Corrosion induced failure of the ductile iron pipes at micro- and nano-levels. Engineering Failure Analysis, 121, 105169. doi: https://doi.org/10.1016/j.engfailanal.2020.105169
- Jawad, M. N., Amouzad Mahdiraji, G., Hajibeigy, M. T. (2020). Performance improvement of sacrificial anode cathodic protection system for above ground storage tank. SN Applied Sciences, 2 (12). doi: https://doi.org/10.1007/s42452-020-03823-7
- Baloyi, T., Maledi, N., Andrews, A. (2022). Corrosion performance of zinc phosphate coatings deposited on AA6061-HDG steel. Materials Chemistry and Physics, 283, 126009. doi: https://doi.org/10.1016/j.matchemphys.2022.126009
- Flø, N. E., Faramarzi, L., Iversen, F., Kleppe, E. R., Graver, B., Bryntesen, H. N., Johnsen, K. (2019). Assessment of material selection for the CO2 absorption process with aqueous MEA solution based on results from corrosion monitoring at Technology Centre Mongstad. International Journal of Greenhouse Gas Control, 84, 91–110. doi: https://doi.org/10.1016/j.ijggc.2019.02.004
- Kaban, A. P. S., Ridhova, A., Priyotomo, G., Elya, B., Maksum, A., Sadeli, Y. et. al. (2021). Development of white tea extract as green corrosion inhibitor in mild steel under 1 M hydrochloric acid solution. Eastern-European Journal of Enterprise Technologies, 2 (6 (110)), 6–20. doi: https://doi.org/10.15587/1729-4061.2021.224435
- Arlan, A. S., Subekti, N., Soedarsono, J. W., Rustandi, A. (2018). Corrosion Inhibition by a Caesalpinia Sappan L Modified Imidazoline for Carbon Steel API 5L Grade X60 in HCl 1M Environment. Materials Science Forum, 929, 158–170. doi: https://doi.org/10.4028/www.scientific.net/msf.929.158
- Soedarsono, J. W., Shihab, M. N., Azmi, M. F., Maksum, A. (2018). Study of curcuma xanthorrhiza extract as green inhibitor for API 5L X42 steel in 1M HCl solution. IOP Conference Series: Earth and Environmental Science, 105, 012060. doi: https://doi.org/10.1088/1755-1315/105/1/012060
- Kadhim, A., Al-Amiery, A. A., Alazawi, R., Al-Ghezi, M. K. S., Abass, R. H. (2021). Corrosion inhibitors. A review. International Journal of Corrosion and Scale Inhibition, 10 (1). doi: https://doi.org/10.17675/2305-6894-2021-10-1-3
- Lin, B., Shao, J., Xu, Y., Lai, Y., Zhao, Z. (2021). Adsorption and corrosion of renewable inhibitor of Pomelo peel extract for mild steel in phosphoric acid solution. Arabian Journal of Chemistry, 14 (5), 103114. doi: https://doi.org/10.1016/j.arabjc.2021.103114
- Verma, C., Quraishi, M. A., Rhee, K. Y. (2021). Present and emerging trends in using pharmaceutically active compounds as aqueous phase corrosion inhibitors. Journal of Molecular Liquids, 328, 115395. doi: https://doi.org/10.1016/j.molliq.2021.115395
- Rustandi, A., Soedarsono, J. W., Suharno, B. (2011). The Use of Mixture of Piper Betle and Green Tea as a Green Corrosion Inhibitor for API X-52 Steel in Aerated 3.5 % NaCl Solution at Various Rotation Rates. Advanced Materials Research, 383-390, 5418–5425. doi: https://doi.org/10.4028/www.scientific.net/amr.383-390.5418
- Azmi, M. F., Soedarsono, J. W. (2018). Study of corrosion resistrance of pipeline API 5L X42 using green inhibitor bawang dayak (Eleutherine americanna Merr.) in 1M HCl. IOP Conference Series: Earth and Environmental Science, 105, 012061. doi: https://doi.org/10.1088/1755-1315/105/1/012061
- Kaban, E. E., Maksum, A., Permana, S., Soedarsono, J. W. (2018). Utilization of secang heartwood (caesalpinia sappan l) as a green corrosion inhibitor on carbon steel (API 5L Gr. B) in 3.5% NaCl environment. IOP Conference Series: Earth and Environmental Science, 105, 012062. doi: https://doi.org/10.1088/1755-1315/105/1/012062
- Kusumastuti, R., Pramana, R. I., Soedarsono, J. W. (2017). The use of morinda citrifolia as a green corrosion inhibitor for low carbon steel in 3.5% NaCl solution. AIP Conference Proceedings. doi: https://doi.org/10.1063/1.4978085
- Soltani, N., Bahrami, A., Pech-Canul, M. I., González, L. A. (2015). Review on the physicochemical treatments of rice husk for production of advanced materials. Chemical Engineering Journal, 264, 899–935. doi: https://doi.org/10.1016/j.cej.2014.11.056
- Daniel-Mkpume, C. C., Aigbodion, V. S., Obikwelu, D. O. N. (2021). Electrochemical analysis and microstructure of value-added functional Zn-ZnO-rice husk ash composite coating of mild steel. Chemical Data Collections, 35, 100767. doi: https://doi.org/10.1016/j.cdc.2021.100767
- Nisar, N., Bhat, J. A. (2020). Experimental investigation of Rice Husk Ash on compressive strength, carbonation and corrosion resistance of reinforced concrete. Australian Journal of Civil Engineering, 19 (2), 155–163. doi: https://doi.org/10.1080/14488353.2020.1838419
- Awizar, D. A., Othman, N. K., Jalar, A., Daud, A. R., Rahman, I. A., Al-Hardan, N. H. (2013). Nanosilicate extraction from rice husk ash as green corrosion inhibitor. International Journal of Electrochemical Science, 8, 1759–1769. Available at: https://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.654.1036&rep=rep1&type=pdf
- Paul Setiawan Kaban, A., Mayangsari, W., Syaiful Anwar, M., Maksum, A., Riastuti, R., Aditiyawarman, T., Wahyuadi Soedarsono, J. (2022). Experimental and modelling waste rice husk ash as a novel green corrosion inhibitor under acidic environment. Materials Today: Proceedings, 62, 4225–4234. doi: https://doi.org/10.1016/j.matpr.2022.04.738
- King, A. D., Birbilis, N., Scully, J. R. (2014). Accurate Electrochemical Measurement of Magnesium Corrosion Rates; a Combined Impedance, Mass-Loss and Hydrogen Collection Study. Electrochimica Acta, 121, 394–406. doi: https://doi.org/10.1016/j.electacta.2013.12.124
- Zhao, Q., Guo, J., Cui, G., Han, T., Wu, Y. (2020). Chitosan derivatives as green corrosion inhibitors for P110 steel in a carbon dioxide environment. Colloids and Surfaces B: Biointerfaces, 194, 111150. doi: https://doi.org/10.1016/j.colsurfb.2020.111150
- Ansari, K. R., Quraishi, M. A., Singh, A. (2015). Isatin derivatives as a non-toxic corrosion inhibitor for mild steel in 20% H2SO4. Corrosion Science, 95, 62–70. doi: https://doi.org/10.1016/j.corsci.2015.02.010
- Khadraoui, A., Khelifa, A., Hadjmeliani, M., Mehdaoui, R., Hachama, K., Tidu, A. et. al. (2016). Extraction, characterization and anti-corrosion activity of Mentha pulegium oil: Weight loss, electrochemical, thermodynamic and surface studies. Journal of Molecular Liquids, 216, 724–731. doi: https://doi.org/10.1016/j.molliq.2016.02.005
- Yilmaz, N., Fitoz, A., Ergun, U., Emregül, K. C. (2016). A combined electrochemical and theoretical study into the effect of 2-((thiazole-2-ylimino)methyl)phenol as a corrosion inhibitor for mild steel in a highly acidic environment. Corrosion Science, 111, 110–120. doi: https://doi.org/10.1016/j.corsci.2016.05.002
- Pereira, M. A., Oliveira, J. E. de, Fonseca, C. S. (2021). Influence of the use of rice husk as source of silica on the sol-gel synthesis of bioglass. Cerâmica, 67 (383), 333–337. doi: https://doi.org/10.1590/0366-69132021673833134
- Bergmann CP, P. P. (2015). Raman Spectroscopy of Iron Oxide of Nanoparticles (Fe3O4). Journal of Material Science & Engineering, 05 (01). doi: https://doi.org/10.4172/2169-0022.1000217
- Hanesch, M. (2009). Raman spectroscopy of iron oxides and (oxy)hydroxides at low laser power and possible applications in environmental magnetic studies. Geophysical Journal International, 177 (3), 941–948. doi: https://doi.org/10.1111/j.1365-246x.2009.04122.x
- Yu, Y., Ramachandran, P. V., Wang, M. C. (2014). Shedding new light on lipid functions with CARS and SRS microscopy. Biochimica et Biophysica Acta (BBA) - Molecular and Cell Biology of Lipids, 1841 (8), 1120–1129. doi: https://doi.org/10.1016/j.bbalip.2014.02.003
- Gupta, A., Kumar, M., Ghosh, P., Swati, Thakur, I. S. (2022). Risk assessment of a municipal extended aeration activated sludge treatment plant using physico-chemical and in vitro bioassay analyses. Environmental Technology & Innovation, 26, 102254. doi: https://doi.org/10.1016/j.eti.2021.102254
- Akalezi, C. O., Maduabuchi, A. C., Enenebeaku, C. K., Oguzie, E. E. (2020). Experimental and DFT evaluation of adsorption and inhibitive properties of Moringa oliefera extract on mild steel corrosion in acidic media. Arabian Journal of Chemistry, 13 (12), 9270–9282. doi: https://doi.org/10.1016/j.arabjc.2020.11.010
- Li, S., Qi, B., Luo, J., Zhuang, Y., Wan, Y. (2021). Ultrafast selective adsorption of pretreatment inhibitors from lignocellulosic hydrolysate with metal-organic frameworks: Performance and adsorption mechanisms. Separation and Purification Technology, 275, 119183. doi: https://doi.org/10.1016/j.seppur.2021.119183
- Chelliah, N. M., Padaikathan, P., Kumar, R. (2019). Evaluation of electrochemical impedance and biocorrosion characteristics of as-cast and T4 heat treated AZ91 Mg-alloys in Ringer’s solution. Journal of Magnesium and Alloys, 7 (1), 134–143. doi: https://doi.org/10.1016/j.jma.2019.01.005
- Verma, C., Olasunkanmi, L. O., Ebenso, E. E., Quraishi, M. A., Obot, I. B. (2016). Adsorption Behavior of Glucosamine-Based, Pyrimidine-Fused Heterocycles as Green Corrosion Inhibitors for Mild Steel: Experimental and Theoretical Studies. The Journal of Physical Chemistry C, 120 (21), 11598–11611. doi: https://doi.org/10.1021/acs.jpcc.6b04429
- Shamsheera, K. O., Anupama, R. P., Abraham, J. (2020). Computational simulation, surface characterization, adsorption studies and electrochemical investigation on the interaction of guar gum with mild steel in HCl environment. Results in Chemistry, 2, 100054. doi: https://doi.org/10.1016/j.rechem.2020.100054
- Noorbakhsh Nezhad, A. H., Davoodi, A., Mohammadi Zahrani, E., Arefinia, R. (2020). The effects of an inorganic corrosion inhibitor on the electrochemical behavior of superhydrophobic micro-nano structured Ni films in 3.5% NaCl solution. Surface and Coatings Technology, 395, 125946. doi: https://doi.org/10.1016/j.surfcoat.2020.125946
- Li, H., Zhang, B., Li, Y., Wu, P., Wang, Y., Xie, M. (2022). Effect of novel green inhibitor on corrosion and chemical mechanical polishing properties of cobalt in alkaline slurry. Materials Science in Semiconductor Processing, 146, 106691. doi: https://doi.org/10.1016/j.mssp.2022.106691
- Bhardwaj, N., Sharma, P., Guo, L., Dagdag, O., Kumar, V. (2022). Molecular dynamic simulation and Quantum chemical calculation of phytochemicals present in Beta vulgaris and electrochemical behaviour of Beta vulgaris peel extract as green corrosion inhibitor for stainless steel (SS-410) in acidic medium. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 632, 127707. doi: https://doi.org/10.1016/j.colsurfa.2021.127707
- Subekti, N., Soedarsono, J. W., Riastuti, R., Sianipar, F. D. (2020). Development of environmental friendly corrosion inhibitor from the extract of areca flower for mild steel in acidic media. Eastern-European Journal of Enterprise Technologies, 2 (6 (104)), 34–45. doi: https://doi.org/10.15587/1729-4061.2020.197875
- Pramana, R. I., Kusumastuti, R., Soedarsono, J. W., Rustandi, A. (2013). Corrosion Inhibition of Low Carbon Steel by Pluchea Indica Less. in 3.5% NaCL Solution. Advanced Materials Research, 785-786, 20–24. doi: https://doi.org/10.4028/www.scientific.net/amr.785-786.20
- Wan, S., Chen, H., Liao, B., Guo, X. (2021). Adsorption and anticorrosion mechanism of glucose-based functionalized carbon dots for copper in neutral solution. Journal of the Taiwan Institute of Chemical Engineers, 129, 289–298. doi: https://doi.org/10.1016/j.jtice.2021.10.001
- Hsissou, R., Dagdag, O., Abbout, S., Benhiba, F., Berradi, M., El Bouchti, M. et. al. (2019). Novel derivative epoxy resin TGETET as a corrosion inhibition of E24 carbon steel in 1.0 M HCl solution. Experimental and computational (DFT and MD simulations) methods. Journal of Molecular Liquids, 284, 182–192. doi: https://doi.org/10.1016/j.molliq.2019.03.180
- Lebrini, M., Lagrenée, M., Vezin, H., Traisnel, M., Bentiss, F. (2007). Experimental and theoretical study for corrosion inhibition of mild steel in normal hydrochloric acid solution by some new macrocyclic polyether compounds. Corrosion Science, 49 (5), 2254–2269. doi: https://doi.org/10.1016/j.corsci.2006.10.029
- Chauhan, D. S., Quraishi, M. A., Srivastava, V., Haque, J., ibrahimi, B. E. (2021). Virgin and chemically functionalized amino acids as green corrosion inhibitors: Influence of molecular structure through experimental and in silico studies. Journal of Molecular Structure, 1226, 129259. doi: https://doi.org/10.1016/j.molstruc.2020.129259
- Shao, H., Yin, X., Zhang, K., Yang, W., Chen, Y., Liu, Y. (2022). N-[2-(3-indolyl)ethyl]-cinnamamide synthesized from cinnamomum cassia presl and alkaloid tryptamine as green corrosion inhibitor for Q235 steel in acidic medium. Journal of Materials Research and Technology, 20, 916–933. doi: https://doi.org/10.1016/j.jmrt.2022.07.122
- Fekkar, G. et. al. (2020). Eco-friendly chamaerops humilis l. Fruit extract corrosion inhibitor for mild steel in 1 M HCL. International Journal of Corrosion and Scale Inhibition. doi: https://doi.org/10.17675/2305-6894-2020-9-2-4
- Gautam, A., Siva, T., Sathiyanarayanan, S., Gobi, K. V., Subasri, R. (2022). Capped inhibitor-loaded halloysite nanoclay-based self-healing silica coatings for corrosion protection of mild steel. Ceramics International, 48 (20), 30151–30163. doi: https://doi.org/10.1016/j.ceramint.2022.06.288
- Fan, J., Zhao, Z., Ding, Z., Liu, J. (2018). Synthesis of different crystallographic FeOOH catalysts for peroxymonosulfate activation towards organic matter degradation. RSC Advances, 8 (13), 7269–7279. doi: https://doi.org/10.1039/c7ra12615h
- Farahati, R., Ghaffarinejad, A., Rezania, H. (Jafar), Mousavi-Khoshdel, S. M., Behzadi, H. (2019). Sulfonated aromatic polyamide as water-soluble polymeric corrosion inhibitor of copper in HCl. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 578, 123626. doi: https://doi.org/10.1016/j.colsurfa.2019.123626
- Ramakrishnan, K., Karthikeyan, S., Rajagopal, D. (2022). 2-Methoxy-4-(4-(((6-nitrobenzothiazol-2-yl)amino)methyl)-1-phenyl-1H-pyrazol-3-yl) phenol as powerful anti-corrosion inhibitor substantiated by Langmuir adsorption studies. Materials Letters, 313, 131823. doi: https://doi.org/10.1016/j.matlet.2022.131823
- Ullah, S., Bustam, M. A., Assiri, M. A., Al-Sehemi, A. G., Gonfa, G., Mukhtar, A. et. al. (2020). Synthesis and characterization of mesoporous MOF UMCM-1 for CO2/CH4 adsorption; an experimental, isotherm modeling and thermodynamic study. Microporous and Mesoporous Materials, 294, 109844. doi: https://doi.org/10.1016/j.micromeso.2019.109844
- Kundu, S., Gupta, A. K. (2006). Arsenic adsorption onto iron oxide-coated cement (IOCC): Regression analysis of equilibrium data with several isotherm models and their optimization. Chemical Engineering Journal, 122 (1-2), 93–106. doi: https://doi.org/10.1016/j.cej.2006.06.002
- Swenson, H., Stadie, N. P. (2019). Langmuir’s Theory of Adsorption: A Centennial Review. Langmuir, 35 (16), 5409–5426. doi: https://doi.org/10.1021/acs.langmuir.9b00154
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2022 Agus Kaban, Wahyu Mayangsari, Mochammad Anwar, Ahmad Maksum, Taufik Aditiyawarman, Johny Soedarsono, Aga Ridhova, Rini Riastuti

This work is licensed under a Creative Commons Attribution 4.0 International License.
The consolidation and conditions for the transfer of copyright (identification of authorship) is carried out in the License Agreement. In particular, the authors reserve the right to the authorship of their manuscript and transfer the first publication of this work to the journal under the terms of the Creative Commons CC BY license. At the same time, they have the right to conclude on their own additional agreements concerning the non-exclusive distribution of the work in the form in which it was published by this journal, but provided that the link to the first publication of the article in this journal is preserved.
A license agreement is a document in which the author warrants that he/she owns all copyright for the work (manuscript, article, etc.).
The authors, signing the License Agreement with TECHNOLOGY CENTER PC, have all rights to the further use of their work, provided that they link to our edition in which the work was published.
According to the terms of the License Agreement, the Publisher TECHNOLOGY CENTER PC does not take away your copyrights and receives permission from the authors to use and dissemination of the publication through the world's scientific resources (own electronic resources, scientometric databases, repositories, libraries, etc.).
In the absence of a signed License Agreement or in the absence of this agreement of identifiers allowing to identify the identity of the author, the editors have no right to work with the manuscript.
It is important to remember that there is another type of agreement between authors and publishers – when copyright is transferred from the authors to the publisher. In this case, the authors lose ownership of their work and may not use it in any way.








