Розкриття досліджень рідкого диму з рисового лушпиння як інгібітора зеленої корозії м'якої сталі під 1 М HCl
DOI:
https://doi.org/10.15587/1729-4061.2022.265086Ключові слова:
рідкий дим, інгібітор зеленої корозії, зола лушпиння рису, хемосорбціяАнотація
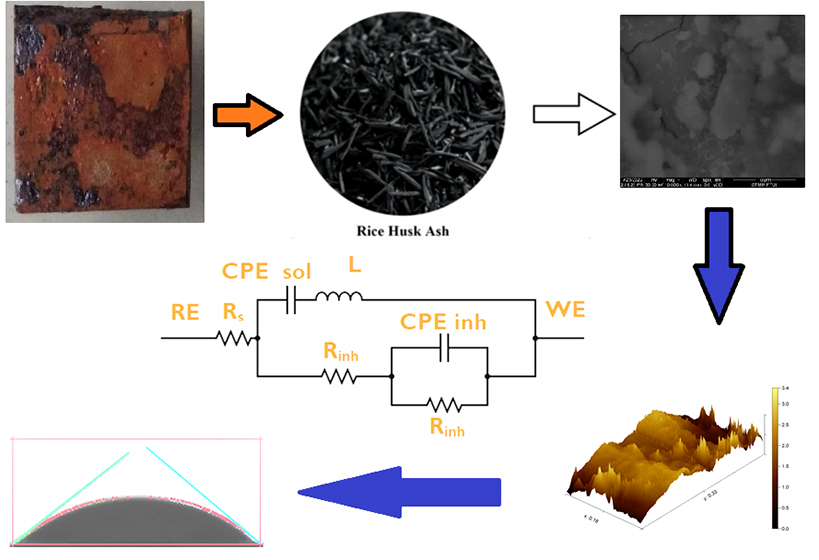
Робота забезпечує більш всебічну розробку рідкого диму із золи рисового лушпиння (ЗРЛ). Примітно, що дослідження зосереджено на взаємодії між первинними молекулами інгібітору та м'якої сталі, включаючи термодинамічний розрахунок та обробку поверхні при додаванні інгібітору. Електрохімічна спектроскопія імпедансу та потенціодинамічна поляризація були використані для оцінки антикорозійного захисту ЗРЛ. Раман-спектроскопію до та після додавання інгібітору ЗРЛ використовували для порівняння адсорбованої функціональної групи інгібіторів. При цьому термодинамічний розрахунок адсорбції інгібітору визначає види адсорбції інгібітора. В результаті процесу адсорбції був застосований скануючий електронний мікроскоп з енергодисперсійним рентгенівським випромінюванням (СЕМ-ЕРВ) у поєднанні з атомно-силовою мікроскопією (АСМ) та тестом контактного кута, щоб виявити обробку поверхні та зміну елементного складу після додавання 80 частин на мільйон інгібітора. Результати ФП та ЕІС показують значне зниження щільності струму при –2,75 мкА·см2 у розчині з концентрацією 80 частин на мільйон за максимальної ефективності інгібування 99,82 %. Краще інгібування корелює з адсорбцією Si-OH, C-C, C-O-C, C=O, складною структурою і -OH при хвильових числах 458, 662, 1095, 1780 та 3530 см-1. Рідкий дим показує значну площу захисної поверхні 0,9982 та високу константу адсорбції (Kадс) 11,648. Розрахункове значення ΔGадс, що дорівнює –6,59 кДж/моль, свідчить про хемосорбцію в природі. У той же час прогнозується, що комбінація розчину з концентрацією 20 та 80 частин на мільйон адсорбується горизонтально, щоб зменшити контакт між розчином та субстратом, як показано в результатах СЕМ та АСМ. Це також збільшує контактний кут та відповідну гідрофобність
Спонсор дослідження
- The author gratefully thanks the Ministry of Research and Technology/National Research and Innovation Agency for the financial support of contract number NKB-1008/UN2.RST/HKP.05.00/2022.
Посилання
- Guillal, A., Ben Seghier, M. E. A., Nourddine, A., Correia, J. A. F. O., Bt Mustaffa, Z., Trung, N.-T. (2020). Probabilistic investigation on the reliability assessment of mid- and high-strength pipelines under corrosion and fracture conditions. Engineering Failure Analysis, 118, 104891. doi: https://doi.org/10.1016/j.engfailanal.2020.104891
- Simmons, M. R. (2008). Report of offshore technology conference (OTC) presentation. Houston, TX: NACE International Oil and Gas Production.
- Gupta, N. K., Verma, C., Salghi, R., Lgaz, H., Mukherjee, A. K., Quraishi, M. A. (2017). New phosphonate based corrosion inhibitors for mild steel in hydrochloric acid useful for industrial pickling processes: experimental and theoretical approach. New Journal of Chemistry, 41 (21), 13114–13129. doi: https://doi.org/10.1039/c7nj01431g
- Aditiyawarman, T., Kaban, A. P. S., Soedarsono, J. W. (2022). A Recent Review of Risk-Based Inspection Development to Support Service Excellence in the Oil and Gas Industry: An Artificial Intelligence Perspective. ASCE-ASME J Risk and Uncert in Engrg Sys Part B Mech Engrg, 9 (1). doi: https://doi.org/10.1115/1.4054558
- Aditiyawarman, T., Soedarsono, J. W., Kaban, A. P. S., Riastuti, R., Rahmadani, H. (2022). The Study of Artificial Intelligent in Risk-Based Inspection Assessment and Screening: A Study Case of Inline Inspection. ASCE-ASME Journal of Risk and Uncertainty in Engineering Systems, Part B: Mechanical Engineering, 9 (1). doi: https://doi.org/10.1115/1.4054969
- Pumps, S. (2010). Materials and Corrosion. Centrifugal Pump Handbook, 227–250. doi: https://doi.org/10.1016/b978-0-7506-8612-9.00008-5
- Wasim, M., Djukic, M. B. (2021). Corrosion induced failure of the ductile iron pipes at micro- and nano-levels. Engineering Failure Analysis, 121, 105169. doi: https://doi.org/10.1016/j.engfailanal.2020.105169
- Jawad, M. N., Amouzad Mahdiraji, G., Hajibeigy, M. T. (2020). Performance improvement of sacrificial anode cathodic protection system for above ground storage tank. SN Applied Sciences, 2 (12). doi: https://doi.org/10.1007/s42452-020-03823-7
- Baloyi, T., Maledi, N., Andrews, A. (2022). Corrosion performance of zinc phosphate coatings deposited on AA6061-HDG steel. Materials Chemistry and Physics, 283, 126009. doi: https://doi.org/10.1016/j.matchemphys.2022.126009
- Flø, N. E., Faramarzi, L., Iversen, F., Kleppe, E. R., Graver, B., Bryntesen, H. N., Johnsen, K. (2019). Assessment of material selection for the CO2 absorption process with aqueous MEA solution based on results from corrosion monitoring at Technology Centre Mongstad. International Journal of Greenhouse Gas Control, 84, 91–110. doi: https://doi.org/10.1016/j.ijggc.2019.02.004
- Kaban, A. P. S., Ridhova, A., Priyotomo, G., Elya, B., Maksum, A., Sadeli, Y. et. al. (2021). Development of white tea extract as green corrosion inhibitor in mild steel under 1 M hydrochloric acid solution. Eastern-European Journal of Enterprise Technologies, 2 (6 (110)), 6–20. doi: https://doi.org/10.15587/1729-4061.2021.224435
- Arlan, A. S., Subekti, N., Soedarsono, J. W., Rustandi, A. (2018). Corrosion Inhibition by a Caesalpinia Sappan L Modified Imidazoline for Carbon Steel API 5L Grade X60 in HCl 1M Environment. Materials Science Forum, 929, 158–170. doi: https://doi.org/10.4028/www.scientific.net/msf.929.158
- Soedarsono, J. W., Shihab, M. N., Azmi, M. F., Maksum, A. (2018). Study of curcuma xanthorrhiza extract as green inhibitor for API 5L X42 steel in 1M HCl solution. IOP Conference Series: Earth and Environmental Science, 105, 012060. doi: https://doi.org/10.1088/1755-1315/105/1/012060
- Kadhim, A., Al-Amiery, A. A., Alazawi, R., Al-Ghezi, M. K. S., Abass, R. H. (2021). Corrosion inhibitors. A review. International Journal of Corrosion and Scale Inhibition, 10 (1). doi: https://doi.org/10.17675/2305-6894-2021-10-1-3
- Lin, B., Shao, J., Xu, Y., Lai, Y., Zhao, Z. (2021). Adsorption and corrosion of renewable inhibitor of Pomelo peel extract for mild steel in phosphoric acid solution. Arabian Journal of Chemistry, 14 (5), 103114. doi: https://doi.org/10.1016/j.arabjc.2021.103114
- Verma, C., Quraishi, M. A., Rhee, K. Y. (2021). Present and emerging trends in using pharmaceutically active compounds as aqueous phase corrosion inhibitors. Journal of Molecular Liquids, 328, 115395. doi: https://doi.org/10.1016/j.molliq.2021.115395
- Rustandi, A., Soedarsono, J. W., Suharno, B. (2011). The Use of Mixture of Piper Betle and Green Tea as a Green Corrosion Inhibitor for API X-52 Steel in Aerated 3.5 % NaCl Solution at Various Rotation Rates. Advanced Materials Research, 383-390, 5418–5425. doi: https://doi.org/10.4028/www.scientific.net/amr.383-390.5418
- Azmi, M. F., Soedarsono, J. W. (2018). Study of corrosion resistrance of pipeline API 5L X42 using green inhibitor bawang dayak (Eleutherine americanna Merr.) in 1M HCl. IOP Conference Series: Earth and Environmental Science, 105, 012061. doi: https://doi.org/10.1088/1755-1315/105/1/012061
- Kaban, E. E., Maksum, A., Permana, S., Soedarsono, J. W. (2018). Utilization of secang heartwood (caesalpinia sappan l) as a green corrosion inhibitor on carbon steel (API 5L Gr. B) in 3.5% NaCl environment. IOP Conference Series: Earth and Environmental Science, 105, 012062. doi: https://doi.org/10.1088/1755-1315/105/1/012062
- Kusumastuti, R., Pramana, R. I., Soedarsono, J. W. (2017). The use of morinda citrifolia as a green corrosion inhibitor for low carbon steel in 3.5% NaCl solution. AIP Conference Proceedings. doi: https://doi.org/10.1063/1.4978085
- Soltani, N., Bahrami, A., Pech-Canul, M. I., González, L. A. (2015). Review on the physicochemical treatments of rice husk for production of advanced materials. Chemical Engineering Journal, 264, 899–935. doi: https://doi.org/10.1016/j.cej.2014.11.056
- Daniel-Mkpume, C. C., Aigbodion, V. S., Obikwelu, D. O. N. (2021). Electrochemical analysis and microstructure of value-added functional Zn-ZnO-rice husk ash composite coating of mild steel. Chemical Data Collections, 35, 100767. doi: https://doi.org/10.1016/j.cdc.2021.100767
- Nisar, N., Bhat, J. A. (2020). Experimental investigation of Rice Husk Ash on compressive strength, carbonation and corrosion resistance of reinforced concrete. Australian Journal of Civil Engineering, 19 (2), 155–163. doi: https://doi.org/10.1080/14488353.2020.1838419
- Awizar, D. A., Othman, N. K., Jalar, A., Daud, A. R., Rahman, I. A., Al-Hardan, N. H. (2013). Nanosilicate extraction from rice husk ash as green corrosion inhibitor. International Journal of Electrochemical Science, 8, 1759–1769. Available at: https://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.654.1036&rep=rep1&type=pdf
- Paul Setiawan Kaban, A., Mayangsari, W., Syaiful Anwar, M., Maksum, A., Riastuti, R., Aditiyawarman, T., Wahyuadi Soedarsono, J. (2022). Experimental and modelling waste rice husk ash as a novel green corrosion inhibitor under acidic environment. Materials Today: Proceedings, 62, 4225–4234. doi: https://doi.org/10.1016/j.matpr.2022.04.738
- King, A. D., Birbilis, N., Scully, J. R. (2014). Accurate Electrochemical Measurement of Magnesium Corrosion Rates; a Combined Impedance, Mass-Loss and Hydrogen Collection Study. Electrochimica Acta, 121, 394–406. doi: https://doi.org/10.1016/j.electacta.2013.12.124
- Zhao, Q., Guo, J., Cui, G., Han, T., Wu, Y. (2020). Chitosan derivatives as green corrosion inhibitors for P110 steel in a carbon dioxide environment. Colloids and Surfaces B: Biointerfaces, 194, 111150. doi: https://doi.org/10.1016/j.colsurfb.2020.111150
- Ansari, K. R., Quraishi, M. A., Singh, A. (2015). Isatin derivatives as a non-toxic corrosion inhibitor for mild steel in 20% H2SO4. Corrosion Science, 95, 62–70. doi: https://doi.org/10.1016/j.corsci.2015.02.010
- Khadraoui, A., Khelifa, A., Hadjmeliani, M., Mehdaoui, R., Hachama, K., Tidu, A. et. al. (2016). Extraction, characterization and anti-corrosion activity of Mentha pulegium oil: Weight loss, electrochemical, thermodynamic and surface studies. Journal of Molecular Liquids, 216, 724–731. doi: https://doi.org/10.1016/j.molliq.2016.02.005
- Yilmaz, N., Fitoz, A., Ergun, U., Emregül, K. C. (2016). A combined electrochemical and theoretical study into the effect of 2-((thiazole-2-ylimino)methyl)phenol as a corrosion inhibitor for mild steel in a highly acidic environment. Corrosion Science, 111, 110–120. doi: https://doi.org/10.1016/j.corsci.2016.05.002
- Pereira, M. A., Oliveira, J. E. de, Fonseca, C. S. (2021). Influence of the use of rice husk as source of silica on the sol-gel synthesis of bioglass. Cerâmica, 67 (383), 333–337. doi: https://doi.org/10.1590/0366-69132021673833134
- Bergmann CP, P. P. (2015). Raman Spectroscopy of Iron Oxide of Nanoparticles (Fe3O4). Journal of Material Science & Engineering, 05 (01). doi: https://doi.org/10.4172/2169-0022.1000217
- Hanesch, M. (2009). Raman spectroscopy of iron oxides and (oxy)hydroxides at low laser power and possible applications in environmental magnetic studies. Geophysical Journal International, 177 (3), 941–948. doi: https://doi.org/10.1111/j.1365-246x.2009.04122.x
- Yu, Y., Ramachandran, P. V., Wang, M. C. (2014). Shedding new light on lipid functions with CARS and SRS microscopy. Biochimica et Biophysica Acta (BBA) - Molecular and Cell Biology of Lipids, 1841 (8), 1120–1129. doi: https://doi.org/10.1016/j.bbalip.2014.02.003
- Gupta, A., Kumar, M., Ghosh, P., Swati, Thakur, I. S. (2022). Risk assessment of a municipal extended aeration activated sludge treatment plant using physico-chemical and in vitro bioassay analyses. Environmental Technology & Innovation, 26, 102254. doi: https://doi.org/10.1016/j.eti.2021.102254
- Akalezi, C. O., Maduabuchi, A. C., Enenebeaku, C. K., Oguzie, E. E. (2020). Experimental and DFT evaluation of adsorption and inhibitive properties of Moringa oliefera extract on mild steel corrosion in acidic media. Arabian Journal of Chemistry, 13 (12), 9270–9282. doi: https://doi.org/10.1016/j.arabjc.2020.11.010
- Li, S., Qi, B., Luo, J., Zhuang, Y., Wan, Y. (2021). Ultrafast selective adsorption of pretreatment inhibitors from lignocellulosic hydrolysate with metal-organic frameworks: Performance and adsorption mechanisms. Separation and Purification Technology, 275, 119183. doi: https://doi.org/10.1016/j.seppur.2021.119183
- Chelliah, N. M., Padaikathan, P., Kumar, R. (2019). Evaluation of electrochemical impedance and biocorrosion characteristics of as-cast and T4 heat treated AZ91 Mg-alloys in Ringer’s solution. Journal of Magnesium and Alloys, 7 (1), 134–143. doi: https://doi.org/10.1016/j.jma.2019.01.005
- Verma, C., Olasunkanmi, L. O., Ebenso, E. E., Quraishi, M. A., Obot, I. B. (2016). Adsorption Behavior of Glucosamine-Based, Pyrimidine-Fused Heterocycles as Green Corrosion Inhibitors for Mild Steel: Experimental and Theoretical Studies. The Journal of Physical Chemistry C, 120 (21), 11598–11611. doi: https://doi.org/10.1021/acs.jpcc.6b04429
- Shamsheera, K. O., Anupama, R. P., Abraham, J. (2020). Computational simulation, surface characterization, adsorption studies and electrochemical investigation on the interaction of guar gum with mild steel in HCl environment. Results in Chemistry, 2, 100054. doi: https://doi.org/10.1016/j.rechem.2020.100054
- Noorbakhsh Nezhad, A. H., Davoodi, A., Mohammadi Zahrani, E., Arefinia, R. (2020). The effects of an inorganic corrosion inhibitor on the electrochemical behavior of superhydrophobic micro-nano structured Ni films in 3.5% NaCl solution. Surface and Coatings Technology, 395, 125946. doi: https://doi.org/10.1016/j.surfcoat.2020.125946
- Li, H., Zhang, B., Li, Y., Wu, P., Wang, Y., Xie, M. (2022). Effect of novel green inhibitor on corrosion and chemical mechanical polishing properties of cobalt in alkaline slurry. Materials Science in Semiconductor Processing, 146, 106691. doi: https://doi.org/10.1016/j.mssp.2022.106691
- Bhardwaj, N., Sharma, P., Guo, L., Dagdag, O., Kumar, V. (2022). Molecular dynamic simulation and Quantum chemical calculation of phytochemicals present in Beta vulgaris and electrochemical behaviour of Beta vulgaris peel extract as green corrosion inhibitor for stainless steel (SS-410) in acidic medium. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 632, 127707. doi: https://doi.org/10.1016/j.colsurfa.2021.127707
- Subekti, N., Soedarsono, J. W., Riastuti, R., Sianipar, F. D. (2020). Development of environmental friendly corrosion inhibitor from the extract of areca flower for mild steel in acidic media. Eastern-European Journal of Enterprise Technologies, 2 (6 (104)), 34–45. doi: https://doi.org/10.15587/1729-4061.2020.197875
- Pramana, R. I., Kusumastuti, R., Soedarsono, J. W., Rustandi, A. (2013). Corrosion Inhibition of Low Carbon Steel by Pluchea Indica Less. in 3.5% NaCL Solution. Advanced Materials Research, 785-786, 20–24. doi: https://doi.org/10.4028/www.scientific.net/amr.785-786.20
- Wan, S., Chen, H., Liao, B., Guo, X. (2021). Adsorption and anticorrosion mechanism of glucose-based functionalized carbon dots for copper in neutral solution. Journal of the Taiwan Institute of Chemical Engineers, 129, 289–298. doi: https://doi.org/10.1016/j.jtice.2021.10.001
- Hsissou, R., Dagdag, O., Abbout, S., Benhiba, F., Berradi, M., El Bouchti, M. et. al. (2019). Novel derivative epoxy resin TGETET as a corrosion inhibition of E24 carbon steel in 1.0 M HCl solution. Experimental and computational (DFT and MD simulations) methods. Journal of Molecular Liquids, 284, 182–192. doi: https://doi.org/10.1016/j.molliq.2019.03.180
- Lebrini, M., Lagrenée, M., Vezin, H., Traisnel, M., Bentiss, F. (2007). Experimental and theoretical study for corrosion inhibition of mild steel in normal hydrochloric acid solution by some new macrocyclic polyether compounds. Corrosion Science, 49 (5), 2254–2269. doi: https://doi.org/10.1016/j.corsci.2006.10.029
- Chauhan, D. S., Quraishi, M. A., Srivastava, V., Haque, J., ibrahimi, B. E. (2021). Virgin and chemically functionalized amino acids as green corrosion inhibitors: Influence of molecular structure through experimental and in silico studies. Journal of Molecular Structure, 1226, 129259. doi: https://doi.org/10.1016/j.molstruc.2020.129259
- Shao, H., Yin, X., Zhang, K., Yang, W., Chen, Y., Liu, Y. (2022). N-[2-(3-indolyl)ethyl]-cinnamamide synthesized from cinnamomum cassia presl and alkaloid tryptamine as green corrosion inhibitor for Q235 steel in acidic medium. Journal of Materials Research and Technology, 20, 916–933. doi: https://doi.org/10.1016/j.jmrt.2022.07.122
- Fekkar, G. et. al. (2020). Eco-friendly chamaerops humilis l. Fruit extract corrosion inhibitor for mild steel in 1 M HCL. International Journal of Corrosion and Scale Inhibition. doi: https://doi.org/10.17675/2305-6894-2020-9-2-4
- Gautam, A., Siva, T., Sathiyanarayanan, S., Gobi, K. V., Subasri, R. (2022). Capped inhibitor-loaded halloysite nanoclay-based self-healing silica coatings for corrosion protection of mild steel. Ceramics International, 48 (20), 30151–30163. doi: https://doi.org/10.1016/j.ceramint.2022.06.288
- Fan, J., Zhao, Z., Ding, Z., Liu, J. (2018). Synthesis of different crystallographic FeOOH catalysts for peroxymonosulfate activation towards organic matter degradation. RSC Advances, 8 (13), 7269–7279. doi: https://doi.org/10.1039/c7ra12615h
- Farahati, R., Ghaffarinejad, A., Rezania, H. (Jafar), Mousavi-Khoshdel, S. M., Behzadi, H. (2019). Sulfonated aromatic polyamide as water-soluble polymeric corrosion inhibitor of copper in HCl. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 578, 123626. doi: https://doi.org/10.1016/j.colsurfa.2019.123626
- Ramakrishnan, K., Karthikeyan, S., Rajagopal, D. (2022). 2-Methoxy-4-(4-(((6-nitrobenzothiazol-2-yl)amino)methyl)-1-phenyl-1H-pyrazol-3-yl) phenol as powerful anti-corrosion inhibitor substantiated by Langmuir adsorption studies. Materials Letters, 313, 131823. doi: https://doi.org/10.1016/j.matlet.2022.131823
- Ullah, S., Bustam, M. A., Assiri, M. A., Al-Sehemi, A. G., Gonfa, G., Mukhtar, A. et. al. (2020). Synthesis and characterization of mesoporous MOF UMCM-1 for CO2/CH4 adsorption; an experimental, isotherm modeling and thermodynamic study. Microporous and Mesoporous Materials, 294, 109844. doi: https://doi.org/10.1016/j.micromeso.2019.109844
- Kundu, S., Gupta, A. K. (2006). Arsenic adsorption onto iron oxide-coated cement (IOCC): Regression analysis of equilibrium data with several isotherm models and their optimization. Chemical Engineering Journal, 122 (1-2), 93–106. doi: https://doi.org/10.1016/j.cej.2006.06.002
- Swenson, H., Stadie, N. P. (2019). Langmuir’s Theory of Adsorption: A Centennial Review. Langmuir, 35 (16), 5409–5426. doi: https://doi.org/10.1021/acs.langmuir.9b00154
##submission.downloads##
Опубліковано
Як цитувати
Номер
Розділ
Ліцензія
Авторське право (c) 2022 Agus Kaban, Wahyu Mayangsari, Mochammad Anwar, Ahmad Maksum, Taufik Aditiyawarman, Johny Soedarsono, Aga Ridhova, Rini Riastuti

Ця робота ліцензується відповідно до Creative Commons Attribution 4.0 International License.
Закріплення та умови передачі авторських прав (ідентифікація авторства) здійснюється у Ліцензійному договорі. Зокрема, автори залишають за собою право на авторство свого рукопису та передають журналу право першої публікації цієї роботи на умовах ліцензії Creative Commons CC BY. При цьому вони мають право укладати самостійно додаткові угоди, що стосуються неексклюзивного поширення роботи у тому вигляді, в якому вона була опублікована цим журналом, але за умови збереження посилання на першу публікацію статті в цьому журналі.
Ліцензійний договір – це документ, в якому автор гарантує, що володіє усіма авторськими правами на твір (рукопис, статтю, тощо).
Автори, підписуючи Ліцензійний договір з ПП «ТЕХНОЛОГІЧНИЙ ЦЕНТР», мають усі права на подальше використання свого твору за умови посилання на наше видання, в якому твір опублікований. Відповідно до умов Ліцензійного договору, Видавець ПП «ТЕХНОЛОГІЧНИЙ ЦЕНТР» не забирає ваші авторські права та отримує від авторів дозвіл на використання та розповсюдження публікації через світові наукові ресурси (власні електронні ресурси, наукометричні бази даних, репозитарії, бібліотеки тощо).
За відсутності підписаного Ліцензійного договору або за відсутністю вказаних в цьому договорі ідентифікаторів, що дають змогу ідентифікувати особу автора, редакція не має права працювати з рукописом.
Важливо пам’ятати, що існує і інший тип угоди між авторами та видавцями – коли авторські права передаються від авторів до видавця. В такому разі автори втрачають права власності на свій твір та не можуть його використовувати в будь-який спосіб.









