Investigation of the mechanism of nickel hydroxide formation from nickel nitrate
DOI:
https://doi.org/10.15587/1729-4061.2023.272673Keywords:
nickel hydroxide, nickel nitrate, two-stage formation mechanism, α-modification, nitrate-doped nickel hydroxide, potentiometry, conductometryAbstract
Nickel hydroxides are widely used as electrochemically active substances in alkaline batteries and hybrid supercapacitors; they can be used for electrocatalysis, in electrochemical sensors, and as pigments. Knowledge of the formation mechanism of nickel hydroxides is necessary for developing and optimizing targeted synthesis methods. The thermal effects of processes in the formation of nickel hydroxide from nitrate were studied by the calorimetry method. The mechanism of precipitate formation was investigated by the method of simultaneous potentiometric (with a glass universal electrode) and conductometric titration. The nickel content in the samples obtained at the determined NaOH/Ni2+ ratios was investigated by the chemical method of trilonometry after preliminary dissolution.
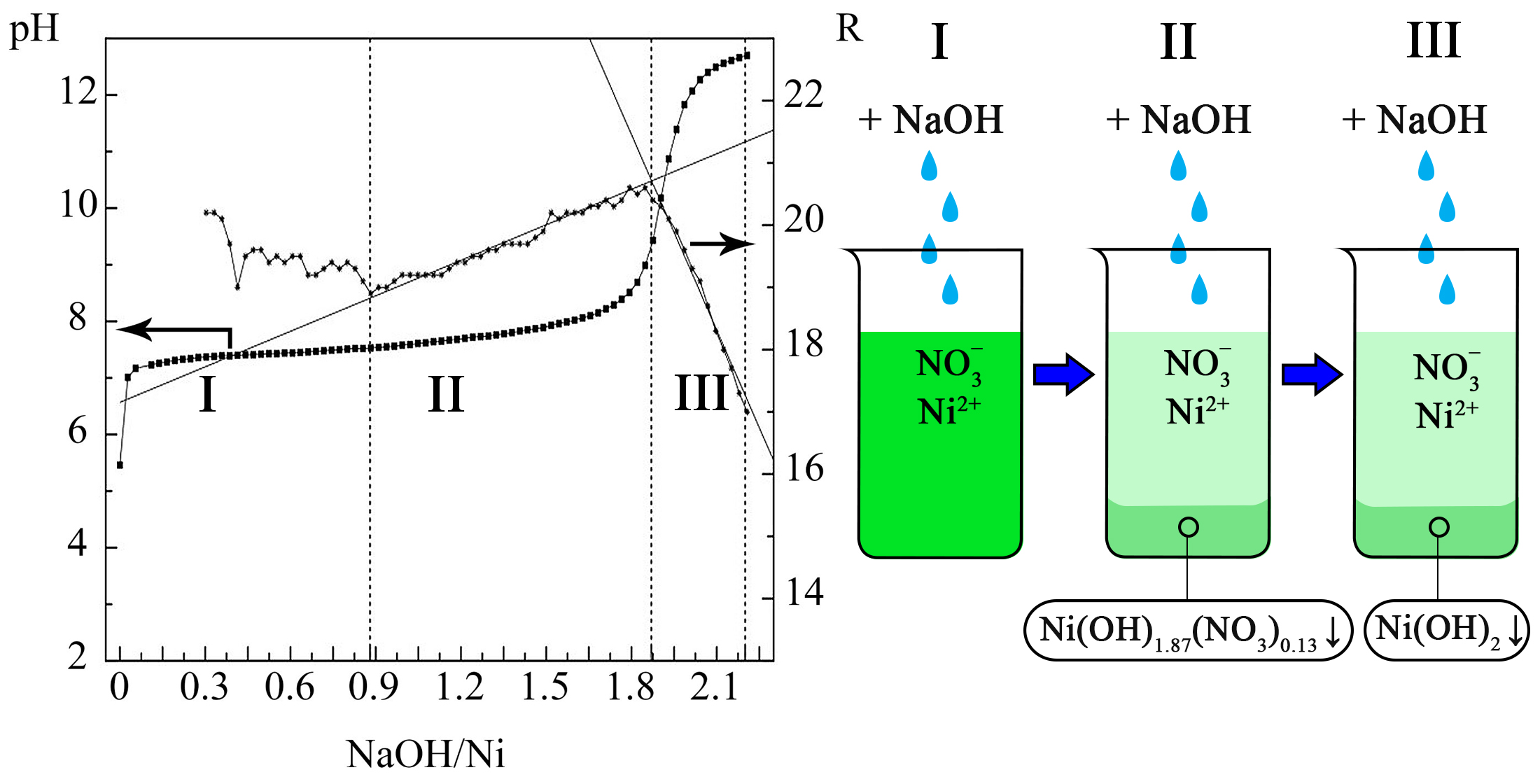
Calorimetric investigations showed that the reaction of nickel nitrate with NaOH was exothermic with ΔНreaction=‒28328.5 J/mol. The exothermic nature of the NaOH dilution process was revealed with ΔНdilution =‒2454 J/mol.
According to the results of potentiometric titration, the formation of a basic salt of the NiOHNO3 type was not detected. Analysis of the results of conductometric titration revealed a two-stage chemical mechanism for the formation of nickel hydroxide from nitrate. At the first stage, which had a high rate, due to the liquid-phase reaction of the nickel cation with the hydroxyl anion, a primary precipitate of the composition Ni(OH)1.87(NO3)0.13 was formed. In the second stage, as a result of a slow topochemical reaction of the primary precipitate with hydroxyl anions, nitrate ions were displaced from the precipitate to form nickel hydroxide. These data are confirmed by the analysis of precipitate obtained at NaOH/Ni2+ ratios of 1.87 and 2.2: the Ni content was 52.95 % and 55.63 %, corresponding to the formulas Ni(OH)1.87(NO3)0.13∙0.68H2O and Ni(OH)2∙0.71H2O. This clearly indicated that the primary precipitate was nitrate-doped α-Ni(OH)2 and the final precipitate corresponded to the α-modification of nickel hydroxide
References
- Hall, D. S., Lockwood, D. J., Bock, C., MacDougall, B. R. (2015). Nickel hydroxides and related materials: a review of their structures, synthesis and properties. Proceedings of the Royal Society A: Mathematical, Physical and Engineering Sciences, 471 (2174), 20140792. doi: https://doi.org/10.1098/rspa.2014.0792
- Vidotti, M., Torresi, R., Torresi, S. I. C. de (2010). Nickel hydroxide modified electrodes: a review study concerning its structural and electrochemical properties aiming the application in electrocatalysis, electrochromism and secondary batteries. Química Nova, 33 (10), 2176–2186. doi: https://doi.org/10.1590/s0100-40422010001000030
- Chen, J., Bradhurst, D. H., Dou, S. X., Liu, H. K. (1999). Nickel Hydroxide as an Active Material for the Positive Electrode in Rechargeable Alkaline Batteries. Journal of The Electrochemical Society, 146 (10), 3606–3612. doi: https://doi.org/10.1149/1.1392522
- Sun, Y.-K., Lee, D.-J., Lee, Y. J., Chen, Z., Myung, S.-T. (2013). Cobalt-Free Nickel Rich Layered Oxide Cathodes for Lithium-Ion Batteries. ACS Applied Materials & Interfaces, 5 (21), 11434–11440. doi: https://doi.org/10.1021/am403684z
- Lang, J.-W., Kong, L.-B., Liu, M., Luo, Y.-C., Kang, L. (2009). Asymmetric supercapacitors based on stabilized α-Ni(OH)2 and activated carbon. Journal of Solid State Electrochemistry, 14 (8), 1533–1539. doi: https://doi.org/10.1007/s10008-009-0984-1
- Lang, J.-W., Kong, L.-B., Wu, W.-J., Liu, M., Luo, Y.-C., Kang, L. (2008). A facile approach to the preparation of loose-packed Ni(OH)2 nanoflake materials for electrochemical capacitors. Journal of Solid State Electrochemistry, 13 (2), 333–340. doi: https://doi.org/10.1007/s10008-008-0560-0
- Aghazadeh, M., Ghaemi, M., Sabour, B., Dalvand, S. (2014). Electrochemical preparation of α-Ni(OH)2 ultrafine nanoparticles for high-performance supercapacitors. Journal of Solid State Electrochemistry, 18 (6), 1569–1584. doi: https://doi.org/10.1007/s10008-014-2381-7
- Zheng, C., Liu, X., Chen, Z., Wu, Z., Fang, D. (2014). Excellent supercapacitive performance of a reduced graphene oxide/Ni(OH)2 composite synthesized by a facile hydrothermal route. Journal of Central South University, 21 (7), 2596–2603. doi: https://doi.org/10.1007/s11771-014-2218-7
- Wang, B., Williams, G. R., Chang, Z., Jiang, M., Liu, J., Lei, X., Sun, X. (2014). Hierarchical NiAl Layered Double Hydroxide/Multiwalled Carbon Nanotube/Nickel Foam Electrodes with Excellent Pseudocapacitive Properties. ACS Applied Materials & Interfaces, 6 (18), 16304–16311. doi: https://doi.org/10.1021/am504530e
- Kotok, V., Kovalenko, V. (2017). The properties investigation of the faradaic supercapacitor electrode formed on foamed nickel substrate with polyvinyl alcohol using. Eastern-European Journal of Enterprise Technologies, 4 (12 (88)), 31–37. doi: https://doi.org/10.15587/1729-4061.2017.108839
- Alshareef, S. F., Alhebshi, N. A., Almashhori, K., Alshaikheid, H. S., Al-hazmi, F. (2022). A Ten-Minute Synthesis of α-Ni(OH)2 Nanoflakes Assisted by Microwave on Flexible Stainless-Steel for Energy Storage Devices. Nanomaterials, 12 (11), 1911. doi: https://doi.org/10.3390/nano12111911
- Wang, Y., Zhang, D., Peng, W., Liu, L., Li, M. (2011). Electrocatalytic oxidation of methanol at Ni–Al layered double hydroxide film modified electrode in alkaline medium. Electrochimica Acta, 56 (16), 5754–5758. doi: https://doi.org/10.1016/j.electacta.2011.04.049
- Huang, W., Li, Z. L., Peng, Y. D., Chen, S., Zheng, J. F., Niu, Z. J. (2005). Oscillatory electrocatalytic oxidation of methanol on an Ni(OH)2 film electrode. Journal of Solid State Electrochemistry, 9 (5), 284–289. doi: https://doi.org/10.1007/s10008-004-0599-5
- Yu, X., Hua, T., Liu, X., Yan, Z., Xu, P., Du, P. (2014). Nickel-Based Thin Film on Multiwalled Carbon Nanotubes as an Efficient Bifunctional Electrocatalyst for Water Splitting. ACS Applied Materials & Interfaces, 6 (17), 15395–15402. doi: https://doi.org/10.1021/am503938c
- Fan, Y., Yang, Z., Cao, X., Liu, P., Chen, S., Cao, Z. (2014). Hierarchical Macro-Mesoporous Ni(OH)2for Nonenzymatic Electrochemical Sensing of Glucose. Journal of The Electrochemical Society, 161 (10), B201–B206. doi: https://doi.org/10.1149/2.0251410jes
- Miao, Y., Ouyang, L., Zhou, S., Xu, L., Yang, Z., Xiao, M., Ouyang, R. (2014). Electrocatalysis and electroanalysis of nickel, its oxides, hydroxides and oxyhydroxides toward small molecules. Biosensors and Bioelectronics, 53, 428–439. doi: https://doi.org/10.1016/j.bios.2013.10.008
- Kovalenko, V., Kotok, V., Yeroshkina, A., Zaychuk, A. (2017). Synthesis and characterisation of dyeintercalated nickelaluminium layereddouble hydroxide as a cosmetic pigment. Eastern-European Journal of Enterprise Technologies, 5 (12 (89)), 27–33. doi: https://doi.org/10.15587/1729-4061.2017.109814
- Kovalenko, V., Kotok, V. (2020). Bifuctional indigocarminintercalated NiAl layered double hydroxide: investigation of characteristics for pigment and supercapacitor application. Eastern-European Journal of Enterprise Technologies, 2 (12 (104)), 30–39. doi: https://doi.org/10.15587/1729-4061.2020.201282
- Kotok, V., Kovalenko, V. (2018). A study of the effect of tungstate ions on the electrochromic properties of Ni(OH)2 films. Eastern-European Journal of Enterprise Technologies, 5 (12 (95)), 18–24. doi: https://doi.org/10.15587/1729-4061.2018.145223
- Kotok, V. A., Kovalenko, V. L. (2019). Non-Metallic Films Electroplating on the Low-Conductivity Substrates: The Conscious Selection of Conditions Using Ni(OH)2 Deposition as an Example. Journal of The Electrochemical Society, 166 (10), D395–D408. doi: https://doi.org/10.1149/2.0561910jes
- Кovalenko, V., Kotok, V., Bolotin, O. (2016). Definition of factors influencing on Ni(OH)2 electrochemical characteristics for supercapacitors. Eastern-European Journal of Enterprise Technologies, 5 (6 (83)), 17–22. doi: https://doi.org/10.15587/1729-4061.2016.79406
- Ramesh, T. N., Kamath, P. V., Shivakumara, C. (2005). Correlation of Structural Disorder with the Reversible Discharge Capacity of Nickel Hydroxide Electrode. Journal of The Electrochemical Society, 152 (4), A806. doi: https://doi.org/10.1149/1.1865852
- Zhao, Y., Zhu, Z., Zhuang, Q.-K. (2005). The relationship of spherical nano-Ni(OH)2 microstructure with its voltammetric behavior. Journal of Solid State Electrochemistry, 10 (11), 914–919. doi: https://doi.org/10.1007/s10008-005-0035-5
- Jayashree, R. S., Kamath, P. V., Subbanna, G. N. (2000). The Effect of Crystallinity on the Reversible Discharge Capacity of Nickel Hydroxide. Journal of The Electrochemical Society, 147 (6), 2029. doi: https://doi.org/10.1149/1.1393480
- Jayashree, R. S., Kamath, P. V. (1999). Factors governing the electrochemical synthesis of α-nickel (II) hydroxide. Journal of Applied Electrochemistry, 29 (4), 449–454. doi: https://doi.org/10.1023/a:1003493711239
- Kotok, V., Kovalenko, V. (2019). Definition of the influence of obtaining method on physical and chemical characteristics of Ni (OH)2 powders. Eastern-European Journal of Enterprise Technologies, 1 (12 (97)), 21–27. doi: https://doi.org/10.15587/1729-4061.2019.156093
- Ramesh, T. N., Kamath, P. V. (2006). Synthesis of nickel hydroxide: Effect of precipitation conditions on phase selectivity and structural disorder. Journal of Power Sources, 156 (2), 655–661. doi: https://doi.org/10.1016/j.jpowsour.2005.05.050
- Rajamathi, M., Vishnu Kamath, P., Seshadri, R. (2000). Polymorphism in nickel hydroxide: role of interstratification. Journal of Materials Chemistry, 10 (2), 503–506. doi: https://doi.org/10.1039/a905651c
- Rajamathi, M., Subbanna, G. N., Kamath, P. V. (1997). On the existence of a nickel hydroxide phase which is neither α nor β. Journal of Materials Chemistry, 7 (11), 2293–2296. doi: https://doi.org/10.1039/a700390k
- Kovalenko, V., Kotok, V. (2019). Anionic carbonate activation of layered (α+β) nickel hydroxide. Eastern-European Journal of Enterprise Technologies, 3 (6 (99)), 44–52. doi: https://doi.org/10.15587/1729-4061.2019.169461
- Kovalenko, V., Kotok, V. (2019). Influence of the carbonate ion on characteristics of electrochemically synthesized layered (α+β) nickel hydroxide. Eastern-European Journal of Enterprise Technologies, 1 (6 (97)), 40–46. doi: https://doi.org/10.15587/1729-4061.2019.155738
- Kovalenko, V., Kotok, V. (2017). Definition of effectiveness of β-Ni(OH)2 application in the alkaline secondary cells and hybrid supercapacitors. Eastern-European Journal of Enterprise Technologies, 5 (6 (89)), 17–22. doi: https://doi.org/10.15587/1729-4061.2017.110390
- Li, J., Luo, F., Tian, X., Lei, Y., Yuan, H., Xiao, D. (2013). A facile approach to synthesis coral-like nanoporous β-Ni(OH) 2 and its supercapacitor application. Journal of Power Sources, 243, 721–727. doi: https://doi.org/10.1016/j.jpowsour.2013.05.172
- Kovalenko, V., Kotok, V. (2018). Influence of ultrasound and template on the properties of nickel hydroxide as an active substance of supercapacitors. Eastern-European Journal of Enterprise Technologies, 3 (12 (93)), 32–39. doi: https://doi.org/10.15587/1729-4061.2018.133548
- Kovalenko, V. L., Kotok, V. A., Sykchin, A., Ananchenko, B. A., Chernyad’ev, A. V., Burkov, A. A. et al. (2020). Al3+ Additive in the Nickel Hydroxide Obtained by High-Temperature Two-Step Synthesis: Activator or Poisoner for Chemical Power Source Application? Journal of The Electrochemical Society, 167 (10), 100530. doi: https://doi.org/10.1149/1945-7111/ab9a2a
- Chen, L., Yang, X., Tian, Y., Wang, Y., Zhao, X., Lei, X., Zhang, F. (2022). Fabrication of β-Ni(OH)2 Particles by Alkaline Etching Layered Double Hydroxides Precursor for Supercapacitor. Frontiers in Energy Research, 9. doi: https://doi.org/10.3389/fenrg.2021.810568
- Hu, M., Yang, Z., Lei, L., Sun, Y. (2011). Structural transformation and its effects on the electrochemical performances of a layered double hydroxide. Journal of Power Sources, 196 (3), 1569–1577. doi: https://doi.org/10.1016/j.jpowsour.2010.08.041
- Córdoba de Torresi, S. I., Provazi, K., Malta, M., Torresi, R. M. (2001). Effect of Additives in the Stabilization of the α Phase of Ni(OH)[sub 2] Electrodes. Journal of The Electrochemical Society, 148 (10), A1179. doi: https://doi.org/10.1149/1.1403731
- Zhang, Z., Zhu, Y., Bao, J., Zhou, Z., Lin, X., Zheng, H. (2012). Structural and electrochemical performance of additives-doped α-Ni(OH)2. Journal of Wuhan University of Technology-Mater. Sci. Ed., 27 (3), 538–541. doi: https://doi.org/10.1007/s11595-012-0500-9
- Sugimoto, A., Ishida, S., Hanawa, K. (1999). Preparation and Characterization of Ni/Al‐Layered Double Hydroxide. Journal of The Electrochemical Society, 146 (4), 1251–1255. doi: https://doi.org/10.1149/1.1391754
- Zhen, F. Z., Quan, J. W., Min, Y. L., Peng, Z., Jun, J. L. (2004). A study on the structure and electrochemical characteristics of a Ni/Al double hydroxide. Metals and Materials International, 10 (5), 485–488. doi: https://doi.org/10.1007/bf03027353
- Liu, B., Wang, X. Y., Yuan, H. T., Zhang, Y. S., Song, D. Y., Zhou, Z. X. (1999). Physical and electrochemical characteristics of aluminium-substituted nickel hydroxide. Journal of Applied Electrochemistry, 29, 853–858. doi: https://doi.org/10.1023/a:1003537900947
- Caravaggio, G. A., Detellier, C., Wronski, Z. (2001). Synthesis, stability and electrochemical properties of NiAl and NiV layered double hydroxides. Journal of Materials Chemistry, 11 (3), 912–921. doi: https://doi.org/10.1039/b004542j
- Li, Y. W., Yao, J. H., Liu, C. J., Zhao, W. M., Deng, W. X., Zhong, S. K. (2010). Effect of interlayer anions on the electrochemical performance of Al-substituted α-type nickel hydroxide electrodes. International Journal of Hydrogen Energy, 35 (6), 2539–2545. doi: https://doi.org/10.1016/j.ijhydene.2010.01.015
- Zhao, Y. (2004). Al-substituted α-nickel hydroxide prepared by homogeneous precipitation method with urea. International Journal of Hydrogen Energy, 29 (8), 889–896. doi: https://doi.org/10.1016/j.ijhydene.2003.10.006
- Lei, L., Hu, M., Gao, X., Sun, Y. (2008). The effect of the interlayer anions on the electrochemical performance of layered double hydroxide electrode materials. Electrochimica Acta, 54 (2), 671–676. doi: https://doi.org/10.1016/j.electacta.2008.07.004
- Faour, A., Mousty, C., Prevot, V., Devouard, B., De Roy, A., Bordet, P. et al. (2012). Correlation among Structure, Microstructure, and Electrochemical Properties of NiAl–CO3 Layered Double Hydroxide Thin Films. The Journal of Physical Chemistry C, 116 (29), 15646–15659. doi: https://doi.org/10.1021/jp300780w
- Solovov, V. A., Nikolenko, N. V., Kovalenko, V. L., Kotok, V. A., Burkov, A. А. et. al. (2018). Synthesis of Ni(II)-Ti(IV) Layered Double Hydroxides Using Coprecipitation At High Supersaturation Method. ARPN Journal of Engineering and Applied Sciences, 13 (24), 9652–9656. Available at: http://www.arpnjournals.org/jeas/research_papers/rp_2018/jeas_1218_7500.pdf
- Kovalenko, V., Kotok, V. (2018). Synthesis of Ni(OH)2 by template homogeneous precipitation for application in the binderfree electrode of supercapacitor. Eastern-European Journal of Enterprise Technologies, 4 (12 (94)), 29–35. doi: https://doi.org/10.15587/1729-4061.2018.140899
- Kovalenko, V., Kotok, V. (2017). Obtaining of Ni–Al layered double hydroxide by slit diaphragm electrolyzer. Eastern-European Journal of Enterprise Technologies, 2 (6 (86)), 11–17. doi: https://doi.org/10.15587/1729-4061.2017.95699
- Vasserman, I. N. (1980). Himicheskoe osazhdenie iz rastvorov. Leningrad: Himiya, 208.
- Burmistr, M. V., Boiko, V. S., Lipko, E. O., Gerasimenko, K. O., Gomza, Yu. P., Vesnin, R. L. et al. (2014). Antifriction and Construction Materials Based on Modified Phenol-Formaldehyde Resins Reinforced with Mineral and Synthetic Fibrous Fillers. Mechanics of Composite Materials, 50 (2), 213–222. doi: https://doi.org/10.1007/s11029-014-9408-0
- Kovalenko, V., Kotok, V. (2017). Selective anodic treatment of W(WC)-based superalloy scrap. Eastern-European Journal of Enterprise Technologies, 1 (5 (85)), 53–58. doi: https://doi.org/10.15587/1729-4061.2017.91205
- Song, Q., Tang, Z., Guo, H., Chan, S. L. I. (2002). Structural characteristics of nickel hydroxide synthesized by a chemical precipitation route under different pH values. Journal of Power Sources, 112 (2), 428–434. doi: https://doi.org/10.1016/s0378-7753(02)00396-8
- Singley, W. J., Carriel, J. T. (1953). A New Method of Studying Basic Metal Salts Applied to Certain Basic Salts of Nickel. Journal of the American Chemical Society, 75 (4), 778–781. doi: https://doi.org/10.1021/ja01100a005
- Kovalenko, V., Kotok, V. (2019). Investigation of characteristics of double Ni–Co and ternary Ni–Co–Al layered hydroxides for supercapacitor application. Eastern-European Journal of Enterprise Technologies, 2 (6 (98)), 58–66. doi: https://doi.org/10.15587/1729-4061.2019.164792
- Paikaray, S., Gomez, M. A., Jim Hendry, M., Essilfie-Dughan, J. (2014). Formation mechanism of layered double hydroxides in Mg2+-, Al3+-, and Fe3+-rich aqueous media: Implications for neutralization in acid leach ore milling. Applied Clay Science, 101, 579–590. doi: https://doi.org/10.1016/j.clay.2014.09.022
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2023 Vadym Kovalenko, Valerii Kotok, Dmitriy Girenko, Mykola Nikolenko, Dmytro Andreiev, Volodymyr Verbitskiy, Volodymyr Medianyk, Svetlana Morozova, Rovil Nafeev

This work is licensed under a Creative Commons Attribution 4.0 International License.
The consolidation and conditions for the transfer of copyright (identification of authorship) is carried out in the License Agreement. In particular, the authors reserve the right to the authorship of their manuscript and transfer the first publication of this work to the journal under the terms of the Creative Commons CC BY license. At the same time, they have the right to conclude on their own additional agreements concerning the non-exclusive distribution of the work in the form in which it was published by this journal, but provided that the link to the first publication of the article in this journal is preserved.
A license agreement is a document in which the author warrants that he/she owns all copyright for the work (manuscript, article, etc.).
The authors, signing the License Agreement with TECHNOLOGY CENTER PC, have all rights to the further use of their work, provided that they link to our edition in which the work was published.
According to the terms of the License Agreement, the Publisher TECHNOLOGY CENTER PC does not take away your copyrights and receives permission from the authors to use and dissemination of the publication through the world's scientific resources (own electronic resources, scientometric databases, repositories, libraries, etc.).
In the absence of a signed License Agreement or in the absence of this agreement of identifiers allowing to identify the identity of the author, the editors have no right to work with the manuscript.
It is important to remember that there is another type of agreement between authors and publishers – when copyright is transferred from the authors to the publisher. In this case, the authors lose ownership of their work and may not use it in any way.








