Одержання вуглеводневих смол суспензійною олігомеризацією фракції С9 піролізу бензину ініційованої амінопероксидами
DOI:
https://doi.org/10.15587/1729-4061.2023.292527Ключові слова:
С9 вуглеводнева смола, суспензійна олігомеризація, N-заміщений амінопероксид, світлий колірАнотація
Об'єкт дослідження – одержання С9 вуглеводневих смол олігомеризацією побічних продуктів нафтопереробки.
У технологіях вуглеводневих смол шляхом вільнорадикальної олігомеризації існує недолік у використанні високих температур та значної тривалості реакції. Одержані продукти мають темний колір, що обмежує їх використання у лакофарбових матеріалах.
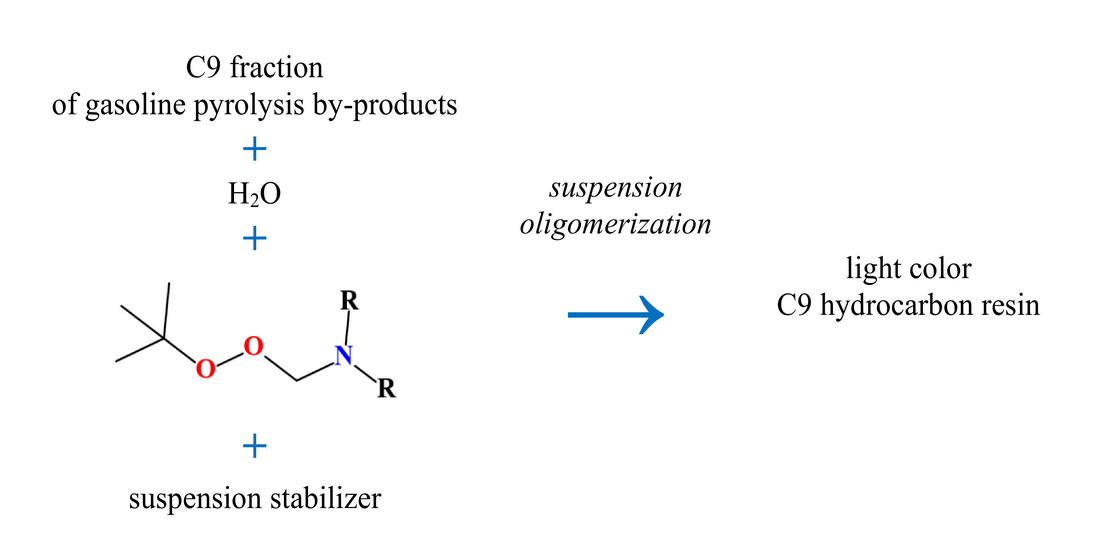
Вивчено технологію вуглеводневих смол, яка полягає у суспензійній олігомеризації вуглеводнів фракції С9 у водному середовищі, при низьких температурах і перемішуванні.
Як ініціатори використовували N-заміщені амінопероксиди, які характеризуються низькою температурою використання. Як сировину використовували фракції С9 рідких продуктів піролізу бензину з вмістом ненасичених сполук – 54,7 %.
Встановлено оптимальні умови: температура – 364 K, тривалість– 180 хв, Re=10120. концентрація ініціатора 0,064 моль/л, частка фракції С9 у реакційній суміші – 25 %. За цих умов одержується світла вуглеводнева смола з показником кольору 20 мг I2/100 мл і температурою розм’якшення – 352 K. Вихід продукту становить 36,0 %.
Застосування амінопероксидних ініціаторів дозволяє проводити суспензійну олігомеризацію вуглеводневої фракції С9 за невисоких температур (303–353 K) і впродовж короткого часу реакції (180 хв). Така технологія олігомеризації дозволяє легко відводити надлишкове тепло і підтримувати ізотермічні умови у зоні реакції. Це запобігає розвитку побічних реакцій окиснення, що спричиняють потемніння продукту.
Результати досліджень дають можливість удосконалити процес олігомеризації вуглеводневої фракції з використанням нових ініціаторів та отримати вуглеводневі смоли світлого кольору. Це дозволить знизити енергетичні затрати на виробництво та покращити характеристики вуглеводневих смол
Посилання
- Speight, J. G. (2020). Handbook of Industrial Hydrocarbon Processes. Elsevier. doi: https://doi.org/10.1016/c2015-0-06314-6
- Mildenberg, R., Zander, M., Collin, G. (1997). Hydrocarbon Resins. VCH Verlagsgesellschaft mbH. doi: https://doi.org/10.1002/9783527614653
- Rahmatpour, A., Ghasemi Meymandi, M. (2021). Large-Scale Production of C9 Aromatic Hydrocarbon Resin from the Cracked-Petroleum-Derived C9 Fraction: Chemistry, Scalability, and Techno-economic Analysis. Organic Process Research & Development, 25 (1), 120–135. doi: https://doi.org/10.1021/acs.oprd.0c00474
- Zohuriaan-Mehr, M. J., Omidian, H. (2000). Petroleum Resins: An Overview. Journal of Macromolecular Science, Part C: Polymer Reviews, 40 (1), 23–49. doi: https://doi.org/10.1081/mc-100100577
- Subtelnyy, R., Zhuravskyi, Y., Kichura, D., Dzinyak, B. (2022). Oligomerization of C9 hydrocarbon fraction initiated by amino peroxides with cyclic substitute. Eastern-European Journal of Enterprise Technologies, 3 (6 (117)), 23–31. doi: https://doi.org/10.15587/1729-4061.2022.259892
- Dzumedzei, M. V., Kucher, R. V., Turovskyi, A. A., Koshovskyi, B. I. (1971). Doslidzhennia kinetyky termichnoho rozpadu azotumisnykh perekysnykh spoluk z tret-alkilnym radykalom. Ukrainskiy himicheskiy zhurnal, 39, 1142–1145.
- Turovskyi, A. A., Dzumudzei, M. V. (1973). Pro kinetyku peredachi lantsiuha cherez azotvmisni perekysy z tret-butylnym radykalom pry polimeryzatsiyi styrolu v masi. Dopovidi NAN Ukrainy, 5, 1106–1108.
- Yang, J., Cao, Z., Qi, Y. (2014). Polymerization of C9 Fraction from Ethylene Cracking Catalyzed by Al3+-Loaded Styrenic Cation Exchange Resin. Asian Journal of Chemistry, 26 (19), 6658–6664. doi: https://doi.org/10.14233/ajchem.2014.17387
- Rahmatpour, A., Soleimani, P., Karamian, S., Dadvand, R. (2023). Use of a cross-linked polystyrene/titanium tetrachloride tightly bound coordination complex as catalyst for the production of petroleum resins. Reaction Chemistry & Engineering, 8 (7), 1583–1597. doi: https://doi.org/10.1039/d2re00429a
- Wang, G. Q., Zhang, W. X., Liang, J. C., Chen, G. Y., Wei, Z. Y., Zhang, L. (2013). Preparation of C5 Petroleum Resins Using Et3NHCl-AlCl3 as Catalyst. Asian Journal of Chemistry, 25 (5), 2829–2832. doi: https://doi.org/10.14233/ajchem.2013.14017
- Subtelnyy, R. O., Kichura, D. B., Dzinyak, B. O. (2022). Synthesis of petroleum resins in the presence of aliphatic aminoperoxides. Voprosy Khimii i Khimicheskoi Tekhnologii, 6, 88–97. doi: https://doi.org/10.32434/0321-4095-2022-145-6-88-97
- Sae‐Ma, N., Praserthdam, P., Panpranot, J., Chaemchuen, S., Dokjamp, S., Suriye, K., Rempel, G. L. (2010). Color improvment of C9 hydrocarbon resin by hydrogenation over 2% Pd/γ‐alumina catalyst: Effect of degree of aromatic rings hydrogenation. Journal of Applied Polymer Science, 117 (5), 2862–2869. doi: https://doi.org/10.1002/app.32189
- Jiang, M., Wei, X., Chen, X., Wang, L., Liang, J. (2020). C9 Petroleum Resin Hydrogenation over a PEG1000-Modified Nickel Catalyst Supported on a Recyclable Fluid Catalytic Cracking Catalyst Residue. ACS Omega, 5 (32), 20291–20298. doi: https://doi.org/10.1021/acsomega.0c02193
- Subtelnyy, R., Kichura, D., Dzinyak, B. (2021). Correlation between the emulsion oligomerization parameters for C9 fraction and the characteristics of hydrocarbon resins. Eastern-European Journal of Enterprise Technologies, 3 (6 (111)), 6–11. doi: https://doi.org/10.15587/1729-4061.2021.232684
- Kovačič, S., Slugovc, C. (2020). Ring-opening Metathesis Polymerisation derived poly(dicyclopentadiene) based materials. Materials Chemistry Frontiers, 4 (8), 2235–2255. doi: https://doi.org/10.1039/d0qm00296h
- Yao, Z., Xu, X., Dong, Y., Liu, X., Yuan, B., Wang, K. et al. (2020). Kinetics on thermal dissociation and oligomerization of dicyclopentadiene in a high temperature & pressure microreactor. Chemical Engineering Science, 228, 115892. doi: https://doi.org/10.1016/j.ces.2020.115892
- Draper, N. R., Smith, H. (1998). Applied Regression Analysis. Wiley Series in Probability and Statistics. doi: https://doi.org/10.1002/9781118625590
##submission.downloads##
Опубліковано
Як цитувати
Номер
Розділ
Ліцензія
Авторське право (c) 2023 Roman Subtelnyy, Yevhenii Zhuravskyi, Bohdan Dzinyak

Ця робота ліцензується відповідно до Creative Commons Attribution 4.0 International License.
Закріплення та умови передачі авторських прав (ідентифікація авторства) здійснюється у Ліцензійному договорі. Зокрема, автори залишають за собою право на авторство свого рукопису та передають журналу право першої публікації цієї роботи на умовах ліцензії Creative Commons CC BY. При цьому вони мають право укладати самостійно додаткові угоди, що стосуються неексклюзивного поширення роботи у тому вигляді, в якому вона була опублікована цим журналом, але за умови збереження посилання на першу публікацію статті в цьому журналі.
Ліцензійний договір – це документ, в якому автор гарантує, що володіє усіма авторськими правами на твір (рукопис, статтю, тощо).
Автори, підписуючи Ліцензійний договір з ПП «ТЕХНОЛОГІЧНИЙ ЦЕНТР», мають усі права на подальше використання свого твору за умови посилання на наше видання, в якому твір опублікований. Відповідно до умов Ліцензійного договору, Видавець ПП «ТЕХНОЛОГІЧНИЙ ЦЕНТР» не забирає ваші авторські права та отримує від авторів дозвіл на використання та розповсюдження публікації через світові наукові ресурси (власні електронні ресурси, наукометричні бази даних, репозитарії, бібліотеки тощо).
За відсутності підписаного Ліцензійного договору або за відсутністю вказаних в цьому договорі ідентифікаторів, що дають змогу ідентифікувати особу автора, редакція не має права працювати з рукописом.
Важливо пам’ятати, що існує і інший тип угоди між авторами та видавцями – коли авторські права передаються від авторів до видавця. В такому разі автори втрачають права власності на свій твір та не можуть його використовувати в будь-який спосіб.









