Determining the possibility of using cold plasma for the oxidation of atmospheric nitrogen into nitrogen oxides and the influence of activating substances on the process
DOI:
https://doi.org/10.15587/1729-4061.2023.293873Keywords:
molecular nitrogen, direct oxidation, cold plasma, nitrogen oxides, nitric acid, plasma torch, activator substance, hydrogen peroxideAbstract
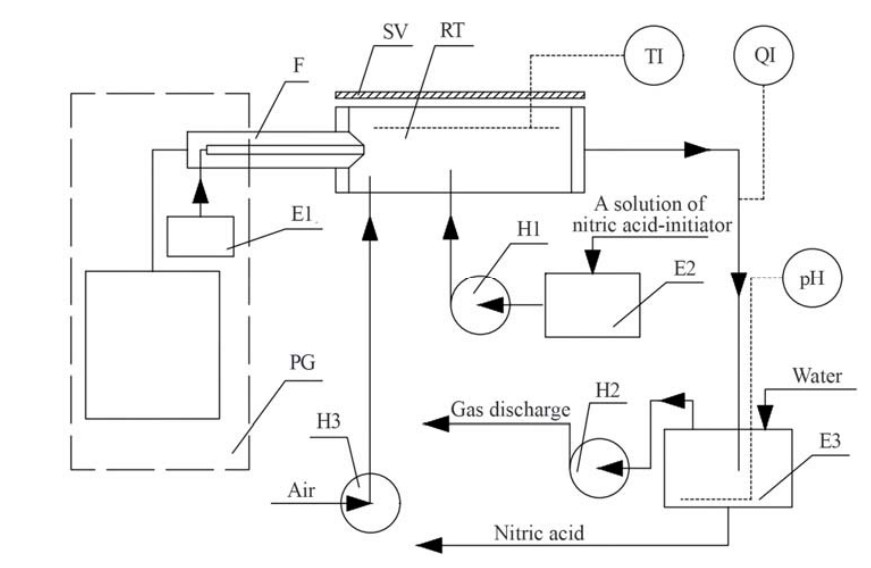
The process of oxidation of molecular nitrogen by high-energy oxidants, such as nitric acid vapor, products of the thermolysis of nitric acid, and hydrogen peroxide, in a cold plasma stream was studied. To implement the process of obtaining nitric acid from atmospheric air using reproductive technology (Zakharov's method), the design of a reactor for obtaining nitrogen oxides by direct oxidation of nitrogen in a cold plasma stream is proposed. At the same time, it was proposed to use the effect of obtaining nitrogen oxides in an air mixture with nitric acid vapors (the Karavaev effect) and during the thermal decomposition of hydrogen peroxide with atmospheric nitrogen (the Nagiev effect). The effectiveness of the use of cold plasma for the oxidation of atmospheric nitrogen was established, which is confirmed by the obtained dependences. It is shown that the amount of nitrogen oxides that are formed depends on the efficiency of the formation of a stable flow of OH- radicals in the plasma flow. It was also found that the amount of nitrogen oxides depends on the parameters of the plasma generator, the composition of the liquid used in the burner, and the amount of air supplied.
The effect of nitric acid, hydrogen peroxide, and alcohols as activators of atmospheric nitrogen oxidation in a high-energy field was revealed. It was determined that when comparing three activator substances, which are able to form OH- radicals during their decomposition, it is hydrogen peroxide that is the most promising activator substance for carrying out the process of atmospheric nitrogen oxidation in the plasma flow.
The amount of nitrogen oxides formed in the cold plasma region is almost independent of the flow rate of the reaction mixture through the reactor and remains almost unchanged in a wide range of changes in flow rates from 30 to 3000 l/h
References
- Nagiev, T. M. (1985). Sopryazhennye reaktsii okisleniya perekis'yu vodovoda. Uspehi himii, 54 (10), 1654–1673.
- Nagiev, M. F., Nagiev, T. M., Aslanov, F. A., Bayramov, V. M., Iskenderov, R. A. (1973). Svyazyvanie azota v vide ego zakisi. DAN SSSR, 213 (5), 1096–1098.
- Karavaev, M. M., Matyshak, V. A. (1998). Geterogenno-kataliticheskoe okislenie azota parami azotnoy kisloty. Himicheskaya promyshlennost', 9, 537–542.
- Zaharov, I. I. (2012). Kvantovo-himicheskoe issledovanie vozmozhnosti foto-himicheskoy aktivatsii molekulyarnogo azota. Teoreticheskaya i eksperimental'naya himiya, 48 (3), 191–195.
- Zakharov, I. I., Ijagbuji, A. A., Tselishtev, A. B. et al. (2014). Ecologically pure technology for the direct oxidation of molecular nitrogen to nitric acid. Advances in Quantum Systems Research, 253–272.
- Crowley, J. N., Carl, S. A. (1997). OH Formation in the Photoexcitation of NO2 beyond the Dissociation Threshold in the Presence of Water Vapor. The Journal of Physical Chemistry A, 101 (23), 4178–4184. doi: https://doi.org/10.1021/jp970319e
- Tselishchev, A., Loriya, M., Boychenko, S., Kudryavtsev, S., Laneckij, V. (2020). Research of change in fraction composition of vehicle gasoline in the modification of its biodethanol in the cavitation field. EUREKA: Physics and Engineering, 5, 12–20. doi: https://doi.org/10.21303/2461-4262.2020.001399
- Zakharov, I. I. (2012). Quantum Chemistry of Nitric Acid: Electronic Structure and Reactivity of its Decomposition Products. Advances in Chemistry Research, 16, 1–51.
- Minaev, B. F., Zakharov, I. I., Zakharova, O. I., Tselishtev, A. B., Filonchook, A. V., Shevchenko, A. V. (2010). Photochemical Water Decomposition in the Troposphere: DFT Study with a Symmetrized Kohn–Sham Formalism. ChemPhysChem, 11 (18), 4028–4034. doi: https://doi.org/10.1002/cphc.201000440
- Zaharov, I. I., Loriya, M. G., Tselishchev, A. B. (2013). Struktura intermediata NOO-N=N-OON pri aktivatsii N2 perekis'yu vodoroda. Kvantovo-himicheskie DFT raschety. Zhurnal strukturnoy himii, 54 (1), 17–24.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2023 Viktor Slobodyanyuk, Andrii Kuzmenko, Serhii Kudriavtsev, Olexii Tselishchev, Maryna Loriia

This work is licensed under a Creative Commons Attribution 4.0 International License.
The consolidation and conditions for the transfer of copyright (identification of authorship) is carried out in the License Agreement. In particular, the authors reserve the right to the authorship of their manuscript and transfer the first publication of this work to the journal under the terms of the Creative Commons CC BY license. At the same time, they have the right to conclude on their own additional agreements concerning the non-exclusive distribution of the work in the form in which it was published by this journal, but provided that the link to the first publication of the article in this journal is preserved.
A license agreement is a document in which the author warrants that he/she owns all copyright for the work (manuscript, article, etc.).
The authors, signing the License Agreement with TECHNOLOGY CENTER PC, have all rights to the further use of their work, provided that they link to our edition in which the work was published.
According to the terms of the License Agreement, the Publisher TECHNOLOGY CENTER PC does not take away your copyrights and receives permission from the authors to use and dissemination of the publication through the world's scientific resources (own electronic resources, scientometric databases, repositories, libraries, etc.).
In the absence of a signed License Agreement or in the absence of this agreement of identifiers allowing to identify the identity of the author, the editors have no right to work with the manuscript.
It is important to remember that there is another type of agreement between authors and publishers – when copyright is transferred from the authors to the publisher. In this case, the authors lose ownership of their work and may not use it in any way.








