Determining patterns of leaching titanium(IV) from the Irshansky deposit ilmenite
DOI:
https://doi.org/10.15587/1729-4061.2024.304661Keywords:
ilmenite concentrate, alkaline leaching, potassium hydroxide, potassium titanate, degree of extractionAbstract
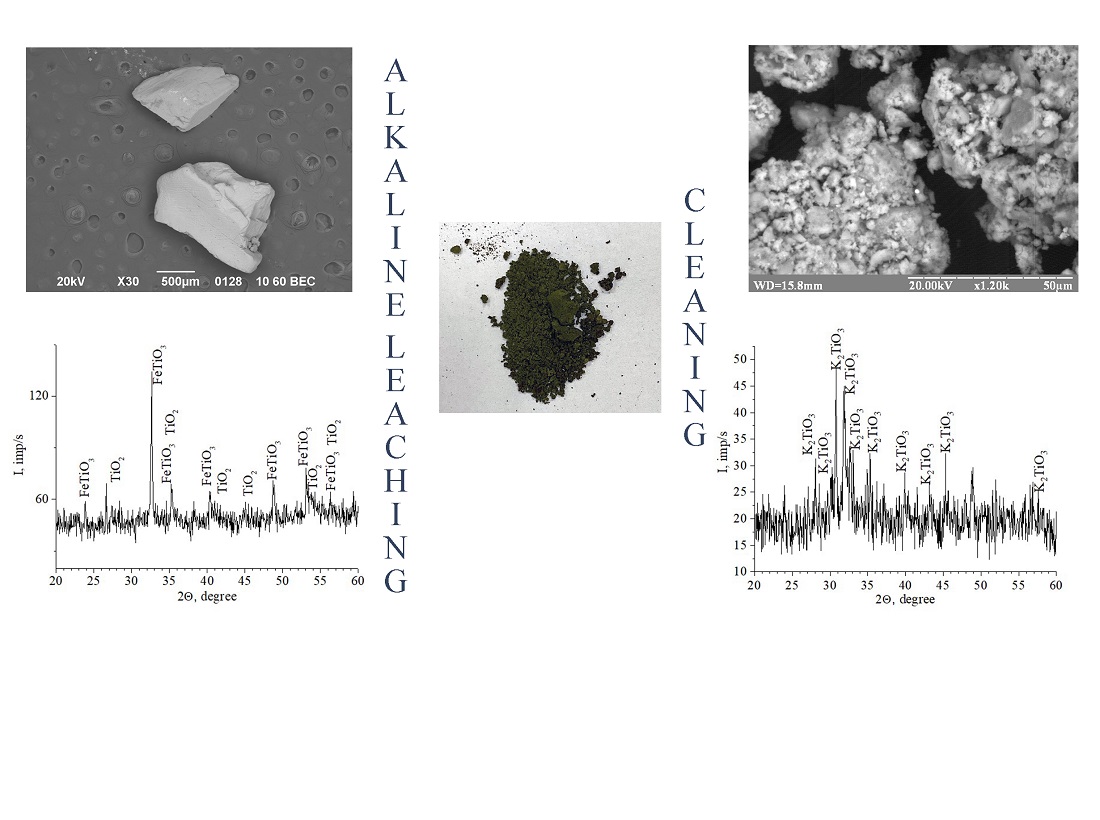
The research object is the ilmenite concentrate from the Irshansky deposit. This study describes an elemental composition of the mineral raw material and confirms its structure using the X-ray diffraction and scanning electron microscopy. Experimental studies have shown that the ilmenite concentrate from the Irshansky deposit has a significant titanium content in terms of titanium dioxide (79 %). Mineral raw materials with such a chemical composition are unique, so there is a need to find alternative methods for its processing. The research demonstrates that the maximum degree of extraction in the process of alkaline leaching of the ilmenite concentrate is achieved under the condition that the average diameter of particles of the mineral raw material should be ≤71 μm. As a result of temperature studies, it has been found that a temperature of 453 K would suffice to obtain potassium titanate at atmospheric pressure. Further temperature increase does not provide for a significant increase in the degree of titanium extraction, and also contributes to the formation of polytitanates of various compositions. The study of the influence of the molar ratio of the starting reagents on the degree of extraction of titanium(IV) from the ilmenite concentrate has showed that the optimal molar ratio between the components corresponds to the stoichiometric one and is 1:2. Increasing the amount of potassium hydroxide in the reaction mixture is impractical as it reduces the yield of potassium titanate, and the final product will have high alkalinity due to excess alkali. The optimal time for alkaline leaching is three hours of continuous heating in a glycerin bath. A further increase in the duration of heating does not lead to an increase in the degree of extraction, which is associated with the diffusion of alkali from the surface of the nucleus into the volume of ilmenite particles due to the formed products of interaction and annihilation of the initial nuclei
References
- Dante, R. C. (2016). Abrasives, ceramic, and inorganic materials. Handbook of Friction Materials and Their Applications, 105–121. https://doi.org/10.1016/b978-0-08-100619-1.00008-0
- Asano, K., Yoneda, H., Agari, Y., Matsumuro, M., Higashi, K. (2015). Thermal and Mechanical Properties of Aluminum Alloy Composite Reinforced with Potassium Hexatitanate Short Fiber. Materials Transactions, 56 (1), 160–166. https://doi.org/10.2320/matertrans.m2014284
- Luo, R., Ni, Y., Li, J., Yang, C., Wang, S. (2011). The mechanical and thermal insulating properties of resin-derived carbon foams reinforced by K2Ti6O13 whiskers. Materials Science and Engineering: A, 528 (4-5), 2023–2027. https://doi.org/10.1016/j.msea.2010.10.106
- Escobedo Bretado, M. A., González Lozano, M. A., Collins Martínez, V., López Ortiz, A., Meléndez Zaragoza, M., Lara, R. H., Moreno Medina, C. U. (2019). Synthesis, characterization and photocatalytic evaluation of potassium hexatitanate (K2Ti6O13) fibers. International Journal of Hydrogen Energy, 44 (24), 12470–12476. https://doi.org/10.1016/j.ijhydene.2018.06.085
- Pysarenko, S., Kaminskyi, O., Chyhyrynets, O., Denysiuk, R., Chernenko, V. (2022). Photocatalytic destruction and adsorptive processes of methylene blue by potassium titanate. Materials Today: Proceedings, 62, 7754–7758. https://doi.org/10.1016/j.matpr.2022.05.476
- Salinas, D., Guerrero, S., Cross, A., Araya, P., Wolf, E. E. (2016). Potassium titanate for the production of biodiesel. Fuel, 166, 237–244. https://doi.org/10.1016/j.fuel.2015.10.127
- Huo, K., Zhao, J., Zhuang, J., Yao, Z., Hu, M., Wang, B. et al. (2024). Hydrothermal synthesis of lepidocrocite-like potassium lithium titanate K0.80Li0.267Ti1.733O4 (KLTO) with superior polarization performance. Chemical Engineering Journal, 482, 148783. https://doi.org/10.1016/j.cej.2024.148783
- Mineral commodity summaries 2022 (2022). US Geological Survey. https://doi.org/10.3133/mcs2022
- Thambiliyagodage, C., Wijesekera, R., Bakker, M. G. (2021). Leaching of ilmenite to produce titanium based materials: a review. Discover Materials, 1 (1). https://doi.org/10.1007/s43939-021-00020-0
- Liu, Y., Qi, T., Chu, J., Tong, Q., Zhang, Y. (2006). Decomposition of ilmenite by concentrated KOH solution under atmospheric pressure. International Journal of Mineral Processing, 81 (2), 79–84. https://doi.org/10.1016/j.minpro.2006.07.003
- Liu, Y., Lü, H., Qi, T., Zhang, Y. (2012). Extraction behaviours of titanium and other impurities in the decomposition process of ilmenite by highly concentrated KOH solution. International Journal of Minerals, Metallurgy, and Materials, 19 (1), 9–14. https://doi.org/10.1007/s12613-012-0508-3
- Nayl, A. A., Awwad, N. S., Aly, H. F. (2009). Kinetics of acid leaching of ilmenite decomposed by KOH. Journal of Hazardous Materials, 168 (2-3), 793–799. https://doi.org/10.1016/j.jhazmat.2009.02.076
- Nayl, A. A., Aly, H. F. (2009). Acid leaching of ilmenite decomposed by KOH. Hydrometallurgy, 97 (1-2), 86–93. https://doi.org/10.1016/j.hydromet.2009.01.011
- Kordzadeh-Kermani, V., Schaffie, M., Hashemipour Rafsanjani, H., Ranjbar, M. (2020). A modified process for leaching of ilmenite and production of TiO2 nanoparticles. Hydrometallurgy, 198, 105507. https://doi.org/10.1016/j.hydromet.2020.105507
- Yousef, L. A. (2017). Uranium Adsorption Using Iron-Titanium Mixed Oxides Separated from Ilmenite Mineral, Black Sands, Rosetta, Egypt. Arab Journal of Nuclear Sciences and Applications, 50 (3), 43–57.
- Amer, A. M. (2002). Alkaline pressure leaching of mechanically activated Rosetta ilmenite concentrate. Hydrometallurgy, 67 (1-3), 125–133. https://doi.org/10.1016/s0304-386x(02)00164-0
- Pysarenko, S. V., Chernenko, V. Yu., Chygyrynets, O. E., Kaminskiy, O. M., Myronyak, M. O. (2021). Alkaline leaching of titanium from ilmenite of Irshansk deposit. Voprosy Khimii i Khimicheskoi Tekhnologii, 6, 51–56. https://doi.org/10.32434/0321-4095-2021-139-6-51-56
- Pysarenko, S. V., Kaminskiy, O. M., Chyhyrynets, O. E., Chernenko, V. Yu., Myroniak, M. O., Shvalahin, V. V. (2022). Thermodynamics of leaching of leukoxenized ilmenite. Voprosy Khimii i Khimicheskoi Tekhnologii, 1, 83–87. https://doi.org/10.32434/0321-4095-2022-140-1-83-87
- Fouda, M. F. R., Amin, R. S., Saleh, H. I., Mousa, H. A. (2010). Extraction of Ultrafine Titania from Black Sands Broaden on the Mediterranean Sea Coast in Egypt by Molten Alkalies. Australian Journal of Basic and Applied Sciences, 4 (9), 4256–4265. Available at: https://www.ajbasweb.com/old/ajbas/2010/4256-4265.pdf
- Subagja, R., Andriyah, L., Hanum Lalasari, L. (2013). Decomposition of ilmenite from Bangka Island – Indonesia with KOH solutions. Asian Transactions on Basic and Applied Sciences, 3 (2), 59–64. Available at: https://citeseerx.ist.psu.edu/document?repid=rep1&type=pdf&doi=8b88ded1cc64a891b3992afc19f77e13fa1710ff
- Parirenyatwa, S., Escudero-Castejon, L., Sanchez-Segado, S., Hara, Y., Jha, A. (2016). Comparative study of alkali roasting and leaching of chromite ores and titaniferous minerals. Hydrometallurgy, 165, 213–226. https://doi.org/10.1016/j.hydromet.2015.08.002
- Pysarenko, S., Kaminskyi, O., Chyhyrynets, O., Denysiuk, R., Anichkina, O., Chernenko, V. (2023). Kinetics of alkaline leaching process of titanium (IV) from ilmenite. Journal of Chemical Technology and Metallurgy, 58 (6), 1146–1152. https://doi.org/10.59957/jctm.v58i6.155
- mp-13133. Materials Explorer. Available at: https://next-gen.materialsproject.org/materials/mp-13133/
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2024 Snizhana Pysarenko, Oleksandr Kaminskyi, Roman Denysiuk, Olena Yevdochenko, Olena Chyhyrynets, Olena Anichkina, Olga Avdieieva, Yuliia Lysetska

This work is licensed under a Creative Commons Attribution 4.0 International License.
The consolidation and conditions for the transfer of copyright (identification of authorship) is carried out in the License Agreement. In particular, the authors reserve the right to the authorship of their manuscript and transfer the first publication of this work to the journal under the terms of the Creative Commons CC BY license. At the same time, they have the right to conclude on their own additional agreements concerning the non-exclusive distribution of the work in the form in which it was published by this journal, but provided that the link to the first publication of the article in this journal is preserved.
A license agreement is a document in which the author warrants that he/she owns all copyright for the work (manuscript, article, etc.).
The authors, signing the License Agreement with TECHNOLOGY CENTER PC, have all rights to the further use of their work, provided that they link to our edition in which the work was published.
According to the terms of the License Agreement, the Publisher TECHNOLOGY CENTER PC does not take away your copyrights and receives permission from the authors to use and dissemination of the publication through the world's scientific resources (own electronic resources, scientometric databases, repositories, libraries, etc.).
In the absence of a signed License Agreement or in the absence of this agreement of identifiers allowing to identify the identity of the author, the editors have no right to work with the manuscript.
It is important to remember that there is another type of agreement between authors and publishers – when copyright is transferred from the authors to the publisher. In this case, the authors lose ownership of their work and may not use it in any way.









