Розробка магнітного активатора для запобігання утворенню накипу на поверхні трубчастих електричних водонагрівачів
DOI:
https://doi.org/10.15587/1729-4061.2025.338091Ключові слова:
магнітне поле, солі жорсткості, енергоефективність, водопідготовка, електронагрівач, магніти, кальцій, магнійАнотація
Об’єктом дослідження були процеси утворення накипу в електричних водонагрівальних системах за використання води підвищеної жорсткості.
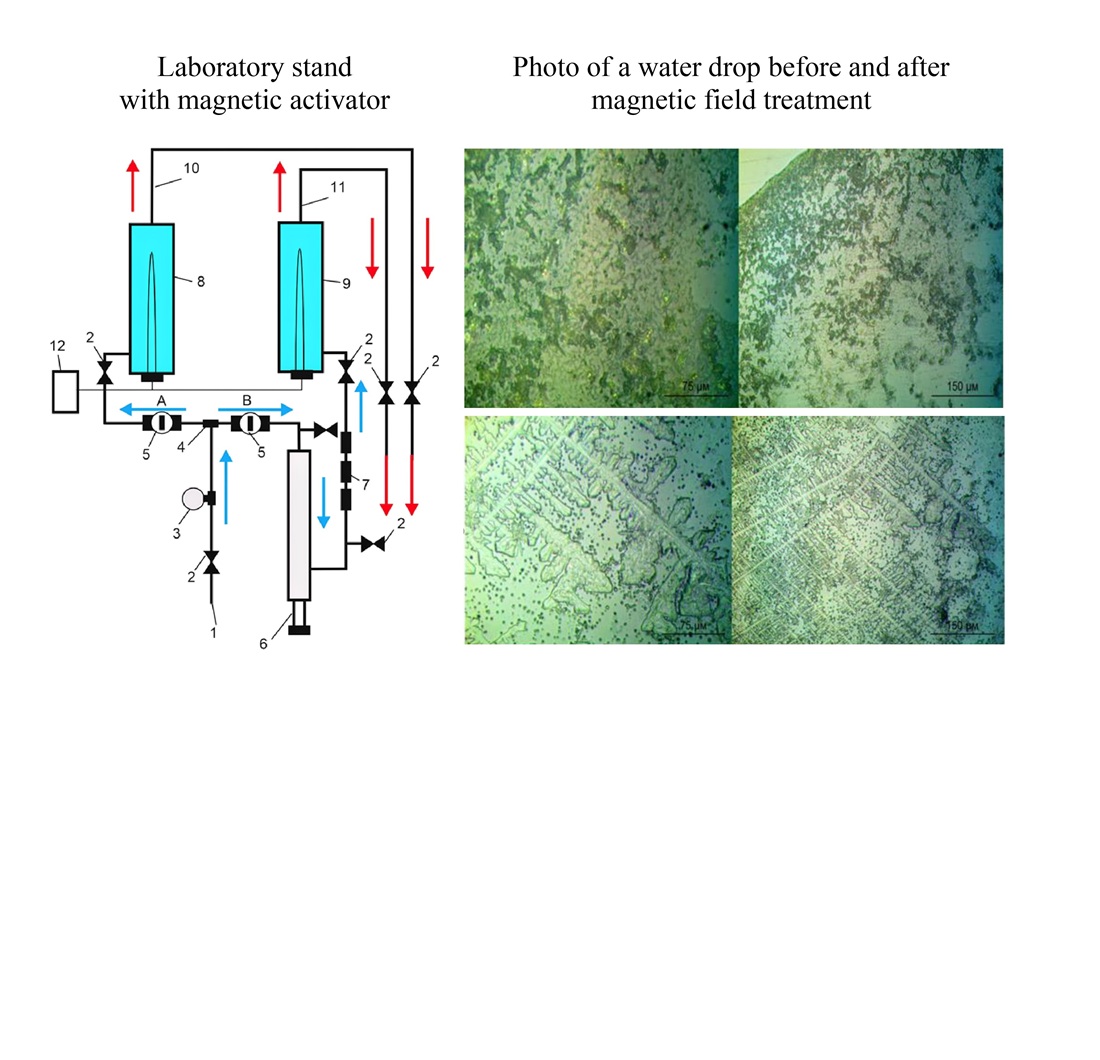
Дослідження присвячено розробці та апробації магнітного активатора для запобігання утворенню накипу на трубчастих електронагрівачах у жорсткій воді без застосування хімічних реагентів.
Пристрій з неодимовими магнітами встановлюється на подаючому трубопроводі водонагрівача та вирішує проблему накипоутворення без використання реагентів. Експеримент тривав 90 днів на лабораторному стенді, що імітував роботу побутового водонагрівача.
Магнітна обробка води знизила загальну жорсткість на 15–20%, вміст іонів Ca2⁺ та Mg2⁺ — на 35–50%, а також сприяла формуванню переважно арагонітної модифікації карбонату кальцію, менш схильної до міцного зчеплення з поверхнею нагріву. Товщина шару накипу на нагрівальному елементі склала близько 0,5 мм проти 2 мм у контрольному зразку, що забезпечило зменшення додаткових енерговитрат із 13% до 3%.
Ефект пояснюється дією магнітного поля на гідратні оболонки іонів жорсткості, що сприяє утворенню менш міцної арагонітної модифікації карбонату кальцію та порушує умови кристалізації стійкого кальциту на нагрівальній поверхні.
Унікальною особливістю пристрою є комбінація внутрішнього та зовнішнього магнітних активаторів на основі неодимових магнітів, що створюють стабільне магнітне поле з високою індукцією, а також наявність відстійника та конструктивних рішень, які запобігають контакту магнітів із водою. Це забезпечило тривалу та спрямовану дію поля на потік води, що підвищило ефективність обробки.
Магнітний активатор ефективний у побутових та промислових системах водонагріву з жорсткою водою, збільшує термін служби обладнання, знижує енергоспоживання та зменшує потребу в хімічних реагентах
Посилання
- Lin, L., Jiang, W., Xu, X., Xu, P. (2020). A critical review of the application of electromagnetic fields for scaling control in water systems: mechanisms, characterization, and operation. Npj Clean Water, 3 (1). https://doi.org/10.1038/s41545-020-0071-9
- Alabi, A., Chiesa, M., Garlisi, C., Palmisano, G. (2015). Advances in anti-scale magnetic water treatment. Environmental Science: Water Research & Technology, 1 (4), 408–425. https://doi.org/10.1039/c5ew00052a
- Naderi, M., Past, V., Mahvi, A. H. (2024). Magnetic treatment as a suppressive method for CaCO3 scale deposition in hard waters in the presence of air bubbles. Desalination and Water Treatment, 318, 100249. https://doi.org/10.1016/j.dwt.2024.100249
- Alimi, F. (2024). Influence of Magnetic Field on Calcium Carbonate Precipitation: A Critical Review. Magnetochemistry, 10 (11), 83. https://doi.org/10.3390/magnetochemistry10110083
- Martínez Moya, S., Boluda Botella, N. (2021). Review of Techniques to Reduce and Prevent Carbonate Scale. Prospecting in Water Treatment by Magnetism and Electromagnetism. Water, 13 (17), 2365. https://doi.org/10.3390/w13172365
- Lin, L., Xu, X., Papelis, C., Xu, P. (2017). Innovative use of drinking water treatment solids for heavy metals removal from desalination concentrate: Synergistic effect of salts and natural organic matter. Chemical Engineering Research and Design, 120, 231–239. https://doi.org/10.1016/j.cherd.2017.02.009
- Padilla González, P., Bautista-Capetillo, C., Ruiz-Canales, A., González-Trinidad, J. et al. (2022). Characterization of Scale Deposits in a Drinking Water Network in a Semi-Arid Region. International Journal of Environmental Research and Public Health, 19 (6), 3257. https://doi.org/10.3390/ijerph19063257
- Poirier, K., Lotfi, M., Garg, K., Patchigolla, K., Anthony, E. J., Faisal, N. H. et al. (2023). A comprehensive review of pre- and post-treatment approaches to achieve sustainable desalination for different water streams. Desalination, 566, 116944. https://doi.org/10.1016/j.desal.2023.116944
- Kozisek, F. (2020). Regulations for calcium, magnesium or hardness in drinking water in the European Union member states. Regulatory Toxicology and Pharmacology, 112, 104589. https://doi.org/10.1016/j.yrtph.2020.104589
- Naderi, M., Nasseri, S., Mahvi, A. H., Mesdaghinia, A., Naddafi, K. (2021). Mechanical trajectory control of water mineral impurities in the electrochemical-magnetic reactor. Desalination and Water Treatment, 238, 67–81. https://doi.org/10.5004/dwt.2021.27756
- Miranzadeh, M. B., Naderi, M., Past, V. (2021). The interaction effect of magnetism on arsenic and iron ions in water. Desalination and Water Treatment, 213, 343–347. https://doi.org/10.5004/dwt.2021.26712
- Gholami, S., Naderi, M., Yousefi, M., Arjmand, M. M. (2019). The electrochemical removal of bacteria from drinking water. Desalination and Water Treatment, 160, 110–115. https://doi.org/10.5004/dwt.2019.24181
- Myśliwiec, D., Szcześ, A., Chibowski, S. (2016). Influence of static magnetic field on the kinetics of calcium carbonate formation. Journal of Industrial and Engineering Chemistry, 35, 400–407. https://doi.org/10.1016/j.jiec.2016.01.026
- Wang, J., Zhang, J., Liang, Y., Xu, Y. (2024). Application of excitation current to characterize the state of calcium carbonate fouling on heat transfer surface under alternating magnetic field. International Journal of Heat and Mass Transfer, 224, 125304. https://doi.org/10.1016/j.ijheatmasstransfer.2024.125304
- Amer, L., Ouhenia, S., Chateigner, D., Gascoin, S., Belabbas, I. (2021). The effect of a magnetic field on the precipitation of calcium carbonate. Applied Physics A, 127 (9). https://doi.org/10.1007/s00339-021-04860-8
- Loureiro, J. B. R., Martins, A. L., Gonçalves, A. S., Souza, B. G. B., Schluter, H. E. P., Santos, H. F. L. et al. (2022). Large-Scale Pipe Flow Experiments for the Evaluation of Nonchemical Solutions for Calcium Carbonate Scaling Inhibition and Control. SPE Journal, 28 (1), 201–214. https://doi.org/10.2118/209476-pa
- Roi, I., Vaskina, I., Jozwiakowski, K., Vaskin, R., Kozii, I. (2020). Influence of the Magnetic Field Gradient on the Efficiency of Magnetic Water Treatment. Advances in Design, Simulation and Manufacturing III. Springer, 387–395. https://doi.org/10.1007/978-3-030-50491-5_37
- Ghernaout, D., Elboughdiri, N. (2020). Magnetic Field Application: An Underappreciated Outstanding Technology. OALib, 7 (1), 1–12. https://doi.org/10.4236/oalib.1106000
- ElMassalami, M., Teixeira, M. S., Elzubair, A. (2025). Investigating the Antiscale Magnetic Treatment Controversy: Insights from the Model Calcium Carbonate Scalant. Scientific Reports, 15 (1). https://doi.org/10.1038/s41598-024-82048-9
- Hamdi, R., Tlili, M. M. (2023). Influence of Foreign Salts and Antiscalants on Calcium Carbonate Crystallization. Crystals, 13 (3), 516. https://doi.org/10.3390/cryst13030516
- Matsuura, T., Okazaki, T., Sazawa, K., Hosoki, A., Ueda, A., Kuramitz, H. (2024). Fiber Optic-Based Portable Sensor for Rapid Evaluation and In Situ Real-Time Sensing of Scale Formation in Geothermal Water. Chemosensors, 12 (9), 171. https://doi.org/10.3390/chemosensors12090171
- Tang, C., Godskesen, B., Aktor, H., Rijn, M. van, Kristensen, J. B., Rosshaug, P. S. et al. (2020). Procedure for Calculating the Calcium Carbonate Precipitation Potential (CCPP) in Drinking Water Supply: Importance of Temperature, Ionic Species and Open/Closed System. Water, 13 (1), 42. https://doi.org/10.3390/w13010042
- Zhang, Z., Jia, Y., Zhao, J. (2020). Effect of Magnesium Ion Concentration on the Scale Inhibition of Heat Exchanger in Circulating Cooling Water under Alternating Electric Field. Applied Sciences, 10 (16), 5491. https://doi.org/10.3390/app10165491
- Van, H. T., Nguyen, L. H., Nguyen, V. D., Nguyen, X. H., Nguyen, T. H., Nguyen, T. V. et al. (2019). Characteristics and mechanisms of cadmium adsorption onto biogenic aragonite shells-derived biosorbent: Batch and column studies. Journal of Environmental Management, 241, 535–548. https://doi.org/10.1016/j.jenvman.2018.09.079
- Chang, B., Li, G., Guo, F., Lu, S., Peng, Y., Hou, J. (2024). Research on Carbon Dioxide-Assisted Electrocoagulation Technology for Treatment of Divalent Cations in Water. Water, 16 (12), 1715. https://doi.org/10.3390/w16121715
- Mekhtiyev, A. D., Sarsikeyev, Ye. Zh., Аtyaksheva, А. V., Аtyaksheva, А. D., Gerassimenko, T. S., Alkina, A. D. (. Method of pre-venting deposits on the inner surface of circulating water pipelines of ferroalloy electric furnace cooling systems. Metalurgija 60 (2021) 3-4, 321-324. https://hrcak.srce.hr/256098
- Mekhtiyev, A., Sarsikeyev, Y., Gerassimenko, T., Alkina, A., Mekhtiyev, R., Neshina, Y., Kirichenko, L. (2024). Development of a magnetic activator to protect an electric water heater against scale formation. Eastern-European Journal of Enterprise Technologies, 6 (1 (132)), 95–102. https://doi.org/10.15587/1729-4061.2024.314957
##submission.downloads##
Опубліковано
Як цитувати
Номер
Розділ
Ліцензія
Авторське право (c) 2025 Aliya Alkina, Ali Mekhtiyev, Yelena Neshina, Yermek Sarsikeyev, Tatyana Gerassimenko, Ruslan Mekhtiyev, Oxana Aldoshina

Ця робота ліцензується відповідно до Creative Commons Attribution 4.0 International License.
Закріплення та умови передачі авторських прав (ідентифікація авторства) здійснюється у Ліцензійному договорі. Зокрема, автори залишають за собою право на авторство свого рукопису та передають журналу право першої публікації цієї роботи на умовах ліцензії Creative Commons CC BY. При цьому вони мають право укладати самостійно додаткові угоди, що стосуються неексклюзивного поширення роботи у тому вигляді, в якому вона була опублікована цим журналом, але за умови збереження посилання на першу публікацію статті в цьому журналі.
Ліцензійний договір – це документ, в якому автор гарантує, що володіє усіма авторськими правами на твір (рукопис, статтю, тощо).
Автори, підписуючи Ліцензійний договір з ПП «ТЕХНОЛОГІЧНИЙ ЦЕНТР», мають усі права на подальше використання свого твору за умови посилання на наше видання, в якому твір опублікований. Відповідно до умов Ліцензійного договору, Видавець ПП «ТЕХНОЛОГІЧНИЙ ЦЕНТР» не забирає ваші авторські права та отримує від авторів дозвіл на використання та розповсюдження публікації через світові наукові ресурси (власні електронні ресурси, наукометричні бази даних, репозитарії, бібліотеки тощо).
За відсутності підписаного Ліцензійного договору або за відсутністю вказаних в цьому договорі ідентифікаторів, що дають змогу ідентифікувати особу автора, редакція не має права працювати з рукописом.
Важливо пам’ятати, що існує і інший тип угоди між авторами та видавцями – коли авторські права передаються від авторів до видавця. В такому разі автори втрачають права власності на свій твір та не можуть його використовувати в будь-який спосіб.









