Determining basic technological parameters for the process of electrochemical extraction of copper from acid sulfate concentrated technological solutions
DOI:
https://doi.org/10.15587/1729-4061.2025.341531Keywords:
cathodic extraction, spent technological solutions, technological parameters, diaphragm and diaphragm-free electrolysisAbstract
This study investigates concentrated model solutions, acid sulfate spent technological solutions (STSs) from surface preparation and coating operations by a number of enterprises, in order to devise unified technologies and to design relevant equipment.
To substantiate the basic parameters for an electrolysis system within the framework of the system approach (Quality Function Deployment), it is shown that regardless of the concept and mechanism of electrochemical transformations, one of the main elements is redox reactions that occur both at the electrode-solution interface and in the solution volume.
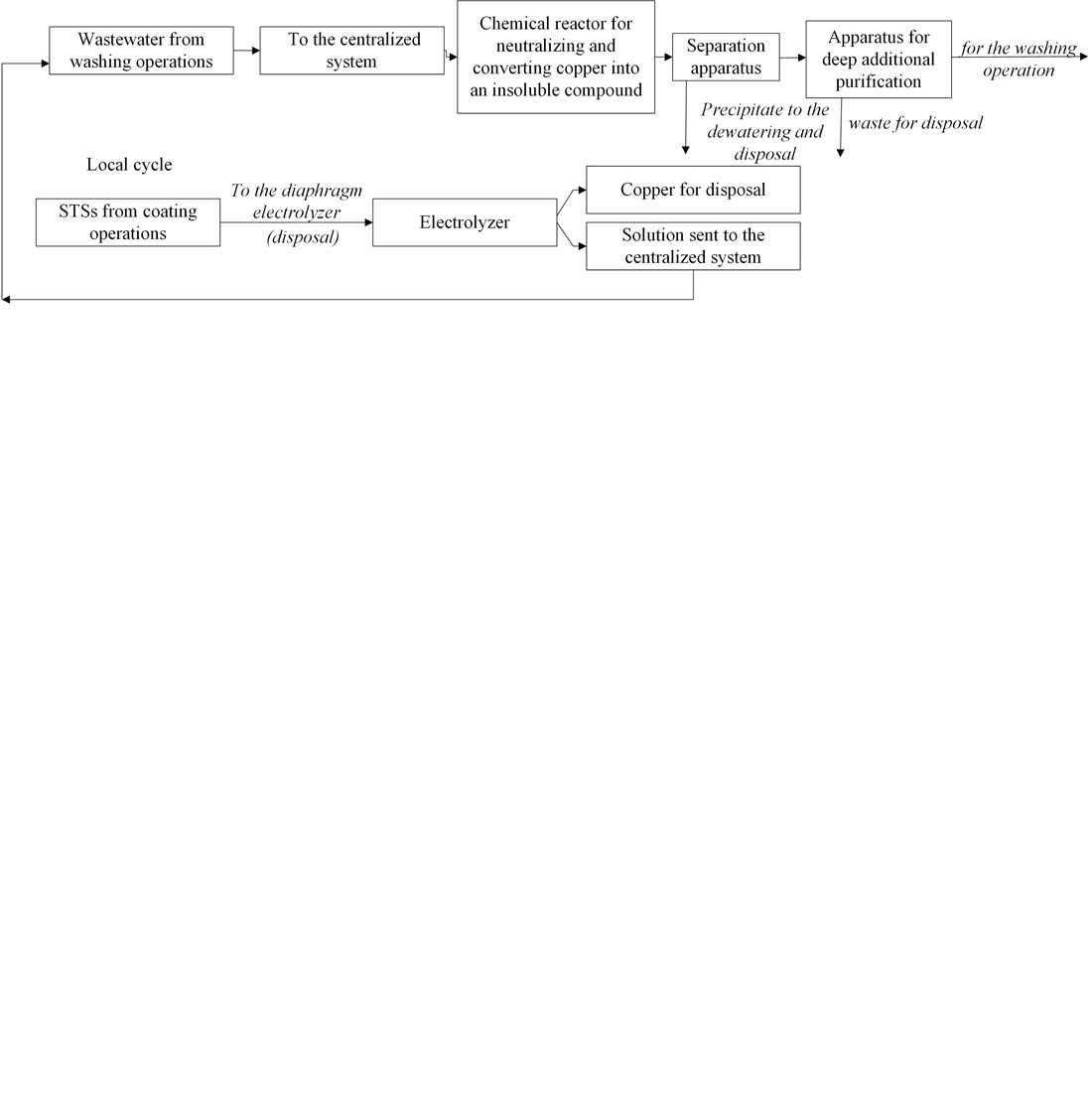
This paper reports experimental studies on electrochemical cathodic extraction of copper from acid sulfate concentrated technological solutions under conditions of non-stationary composition and changes in the properties of STSs. The basic technological parameters of the electrolysis process have been defined; a cathodic extraction installation of metal (copper) has been designed.
To adapt the installation to changes in technological parameters and to avoid the formation of by-products together with the main product (copper), installation and dismantling of cathodes and diaphragms are implied. To eliminate secondary contamination of STS, it is proposed to abandon the use of reagents in local cycles at all stages of STS purification in favor of electrochemical technology.
The kinetic data reported here (current density, current consumption/1 mol, deposition rate) make it possible to define the basic principles of control and regulation of the electrolysis process. The pH and Eh values make it possible to adjust the type of precipitate (foil, precipitate containing foreign substances), as well as determine the purpose of the technological process (regeneration, disposal).
It is recommended to use diaphragm-free electrolysis in local regeneration cycles and diaphragm electrolysis in local disposal cycles
References
- Abidli, A., Huang, Y., Ben Rejeb, Z., Zaoui, A., Park, C. B. (2022). Sustainable and efficient technologies for removal and recovery of toxic and valuable metals from wastewater: Recent progress, challenges, and future perspectives. Chemosphere, 292, 133102. https://doi.org/10.1016/j.chemosphere.2021.133102
- Fylypchuk, V. L., Shatalov, O. S. (2014). Bezpeka ekspluatatsiyi ustanovok dlia elektrokhimichnoho ochyshchennia stichnykh vod promyslovykh pidpryiemstv. Visnyk NUVHP, 1 (65), 436–445. Available at: https://ep3.nuwm.edu.ua/1431/1/Vt6551.pdf
- Grebenyuk, V. D., Linkov, Ν. Α., Linkov, V. M. (1998). Removal of Ni and Cu ions from aqueous solutions by means of a hybrid electrosorption/electrodialysis process. Water SA, 24 (2), 123–127. Available at: https://www.wrc.org.za/wp-content/uploads/mdocs/WaterSA_1998_02_apr98_p123.pdf
- Mourdikoudis, S., Dominguez‐Benetton, X. (2025). Physicochemical vs Electrochemical Technologies for Metal Recovery – Main Insights, Comparison, Complementarity and Challenges. Chemistry–Methods, 5 (3). https://doi.org/10.1002/cmtd.202400046
- Dermentzis, K. I., Marmanis, D. I., Christoforidis, A. K., Stergiopoulos, D. K. (2016). Electrochemical recovery of metallic copper from galvanic effluents. 13th Intern. Conf. PHYSICAL CHEMISTRY 2016. Belgrade. Available at: https://www.researchgate.net/publication/344132075_Electrochemical_recovery_of_metallic_copper_from_galvanic_effluents
- Dzyazko, Yu., Atamanyuh, V. (2004). Electrodionization method and its perspectives for natural and waste waters purification from heavy metals ions. Available at: https://ekmair.ukma.edu.ua/server/api/core/bitstreams/7894a973-6d26-46b3-9d0f-f1ed6ff3076b/content
- Stergiopoulos, D., Dermentzis, K., Spanos, T., Giannakoudakis, P., Agapiou, A., Stylianou, M. (2019). Combined electrocoagulation/electrowinning process for recovery of metallic copper from electroplating effluents. Journal of Engineering Science and Technology Review, 12 (3). Available at: https://www.researchgate.net/publication/344112550_Combined_electrocoagulationelectrowinning_process_for_recovery_of_metallic_copper_from_electroplating_effluents
- Fylypchuk, V. (2002). Elektrokhimichna zmina okysno-vidnovnoho potentsialu pry ochyshchenni stichnykh vod. Visnyk ternopilskoho derzhavnoho tekhnichnoho universytetu, 7 (4), 131–137. Available at: https://elartu.tntu.edu.ua/bitstream/lib/42474/2/TSTUSJ_2002v7n4_Filipchuk_V-Electrochemical_changing_131-137.pdf
- Antropov, L. I. (1993). Teoretychna elektrokhimiya. Kyi: Lybid, 544.
- Yatskov, M. V., Korchyk, N. M., Kyryliuk, S. V. (2024). Ochyshchennia kontsentrovanykh stichnykh vod halvanichnoho vyrobnytstva u kombinovanykh systemakh. Rivne: O. Zen, 200.
- Yatskov, M., Korchyk, N., Prorok, O. (2019). Developing a technology for processing cuprum containing wastes from galvanic production aimed at their further use. Eastern-European Journal of Enterprise Technologies, 6 (10 (102)), 32–41. https://doi.org/10.15587/1729-4061.2019.186620
- Yatskov, M. V., Korchyk, N. M., Prorok, O. A., (2017). Research of physico-chemical properties for high-concentrated suspensions from galvanic manufactures in the reagent slims form. Visnyk NUVHP, 3 (79), 60–67. Available at: http://nbuv.gov.ua/UJRN/Vnuvgp_tekhn_2017_3_9
- Jackowska, K., Krysiński, P. (2020). Applied Electrochemistry. De Gruyter Brill. https://doi.org/10.1515/9783110600834
- Volkov, S. V., Kozin, L. F., Omelchuk, A. O. (2005). Deiaki problemy suchasnoi elektrokhimiyi. Ukrainskyi khimichnyi zhurnal, 71 (7), 3–32. Available at: https://ucj.org.ua/index.php/journal/issue/download/190/7-2005
- Stezeryanskii, E. A. (2024). Electrochemical redox reactions of hexamethylenteramine tetraiodide. Modern Aspects of Electrochemistry. Kyiv: MPBP «Hordon», 120–121. https://doi.org/10.33609/elchimcongr.2024.09.1-210
- Cesiulis, H., Tsyntsaru, N. (2023). Eco-Friendly Electrowinning for Metals Recovery from Waste Electrical and Electronic Equipment (WEEE). Coatings, 13 (3), 574. https://doi.org/10.3390/coatings13030574
- Chen, L., Zhang, G., Liu, H., Miao, S., Chen, Q., Lan, H., Qu, J. (2024). Efficient Metal Recovery from Industrial Wastewater: Potential Oscillation and Turbulence Mode for Electrochemical System. Engineering, 38, 184–193. https://doi.org/10.1016/j.eng.2023.12.002
- Fu, F., Wang, Q. (2011). Removal of heavy metal ions from wastewaters: A review. Journal of Environmental Management, 92 (3), 407–418. https://doi.org/10.1016/j.jenvman.2010.11.011
- Zeng, H., Liu, S., Chai, B., Cao, D., Wang, Y., Zhao, X. (2016). Enhanced Photoelectrocatalytic Decomplexation of Cu–EDTA and Cu Recovery by Persulfate Activated by UV and Cathodic Reduction. Environmental Science & Technology, 50 (12), 6459–6466. https://doi.org/10.1021/acs.est.6b00632
- Yatskov, M. V., Korchyk, N. M., Shchuhailov, V. S., Mysina, O. I. (2001). Pat. No. 35505 A UA. Sposib ochyshchennia stichnykh vod vyrobnytstva drukovanykh plat vid orhanichnykh domishok. No. 99105840; declareted: 26.10.1999; published: 15.03.2001. Available at: https://base.nipo.gov.ua/searchInvRevoke/search.php?action=showsearchresults&page=1&lang=ukr&qp=claims_per_page%3D10%3Bch_0%3Don%3Bqe0%3D35505%3B
- Chan, L.-K., Wu, M.-L. (2002). Quality Function Deployment: A Comprehensive Review of Its Concepts and Methods. Quality Engineering, 15 (1), 23–35. https://doi.org/10.1081/qen-120006708
- Laboratorna robota No. 10 Fotometrychnyi analiz. Vyznachennia vmistu midi (II) u rozch (2020). Analitychna khimiya. Chernihiv: NU «Chernihivska politekhnika», 59–64. Available at: https://ir.stu.cn.ua/bitstream/handle/123456789/20292/%D0%90%D0%BD%D0%B0%D0%BB%D1%96%D1%82%D0%B8%D1%87%D0%BD%D0%B0%20%20%D1%85%D1%96%D0%BC%D1%96%D1%8F..pdf?sequence=1&isAllowed=y/
- Korchyk, N. M., Budenkova, N. M., Sen, O. M. (2013). Elektrokhimichni protsesy vyluchennia metaliv z vidkhodiv halvanichnoho vyrobnytstva. Visnyk NUVHP, 3 (63), 133–141.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2025 Mykola Yatskov, Natalia Korchyk, Nadia Budenkova, Oksana Mysina, Serhii Kovalchuk

This work is licensed under a Creative Commons Attribution 4.0 International License.
The consolidation and conditions for the transfer of copyright (identification of authorship) is carried out in the License Agreement. In particular, the authors reserve the right to the authorship of their manuscript and transfer the first publication of this work to the journal under the terms of the Creative Commons CC BY license. At the same time, they have the right to conclude on their own additional agreements concerning the non-exclusive distribution of the work in the form in which it was published by this journal, but provided that the link to the first publication of the article in this journal is preserved.
A license agreement is a document in which the author warrants that he/she owns all copyright for the work (manuscript, article, etc.).
The authors, signing the License Agreement with TECHNOLOGY CENTER PC, have all rights to the further use of their work, provided that they link to our edition in which the work was published.
According to the terms of the License Agreement, the Publisher TECHNOLOGY CENTER PC does not take away your copyrights and receives permission from the authors to use and dissemination of the publication through the world's scientific resources (own electronic resources, scientometric databases, repositories, libraries, etc.).
In the absence of a signed License Agreement or in the absence of this agreement of identifiers allowing to identify the identity of the author, the editors have no right to work with the manuscript.
It is important to remember that there is another type of agreement between authors and publishers – when copyright is transferred from the authors to the publisher. In this case, the authors lose ownership of their work and may not use it in any way.








