Determination of the efficiency and selectivity of anodic dissolution of a heat-resistant rhenium-containing superalloy in chloride-containing media with sulfuric or methanesulfonic acids
DOI:
https://doi.org/10.15587/1729-4061.2025.342421Keywords:
superalloy recycling, anodic dissolution, rhenium recovery, nickel-based superalloyAbstract
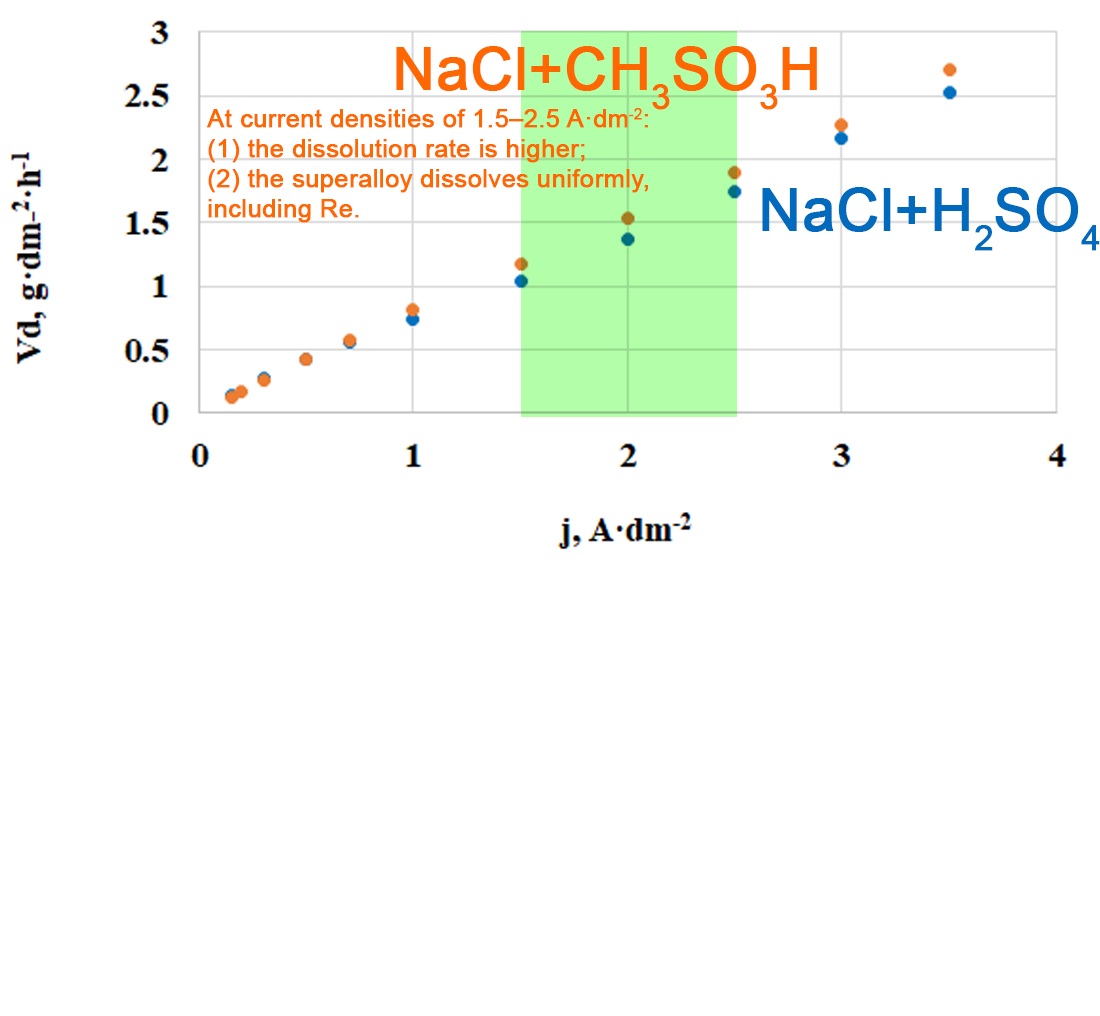
The object of this study is the electrochemical anodic dissolution of a heat-resistant nickel-based superalloy containing rhenium and other alloying elements in acidic electrolytes containing sodium chloride. The investigated alloy was obtained from scrap of high-temperature equipment. The anodic dissolution of the superalloy was studied in two acidic media: sulfuric and methanesulfonic acids. A comparative analysis of cyclic voltammetry and galvanostatic experiments was carried out. In sulfuric acid electrolyte, anodic processes proceed more vigorously, as indicated by higher current densities. However, this method records not only the dissolution currents of metals but also side processes such as anodic oxygen evolution and re-oxidation of dissolved ions. Under galvanostatic conditions, which allow direct determination of alloy mass loss, it was shown that methanesulfonic acid with sodium chloride provides a higher dissolution rate despite the medium's lower conductivity. This effect is explained by the higher solubility and stability of the methanesulfonates of the alloying components (Cr, Al, Nb, Ta, Re), which reduce the tendency of the surface to repassivate. In the H2SO4 + NaCl medium, dissolution proceeds more uniformly but at lower mass efficiency, attributed to the formation of poorly soluble sulfates. In the methanesulfonate electrolyte, within the current density range of 1.5–2.5 A·dm-2, the ratios of Ni, Cr, Co, W, and Re were closest to those in the original alloy, while rhenium was detected in solution, unlike in the sulfuric medium. The obtained results can be applied to optimize the initial stage of superalloy recycling and to develop electrochemical technologies for the recovery of strategically important metals from industrial waste
References
- Miracle, D. B., Senkov, O. N. (2017). A critical review of high entropy alloys and related concepts. Acta Materialia, 122, 448–511. https://doi.org/10.1016/j.actamat.2016.08.081
- Pollock, T. M., Tin, S. (2006). Nickel-Based Superalloys for Advanced Turbine Engines: Chemistry, Microstructure and Properties. Journal of Propulsion and Power, 22 (2), 361–374. https://doi.org/10.2514/1.18239
- Chen, J., Zhou, X., Wang, W., Liu, B., Lv, Y., Yang, W. et al. (2018). A review on fundamental of high entropy alloys with promising high–temperature properties. Journal of Alloys and Compounds, 760, 15–30. https://doi.org/10.1016/j.jallcom.2018.05.067
- Diwahar, D., Manivachakan, V., Syed, R. B. (2025). An Overview of Rare Earth-Doped Ceramic Thermal Barrier Coatings for High-Temperature Performance of Nickel-Based Superalloys. High Temperature Corrosion of Materials, 102 (4). https://doi.org/10.1007/s11085-025-10340-8
- Zhou, Y., Zhao, X., Fan, Y., Yue, Q., Xia, W., Pan, Q. et al. (2025). Composition Optimization in Alloy Design for Nickel-Based Single Crystal Superalloy: A Review. Metals, 15 (7), 793. https://doi.org/10.3390/met15070793
- Darolia, R. (2018). Development of strong, oxidation and corrosion resistant nickel-based superalloys: critical review of challenges, progress and prospects. International Materials Reviews, 64 (6), 355–380. https://doi.org/10.1080/09506608.2018.1516713
- Babiy, K. V., Bubnova, O. A., Malieiev, Ye. V., Riumina, D. M., Levchenko, K. S., Ikol, O. O. (2024). Resursy stratehichnykh korysnykh kopalyn Ukrainy. Dnipro: PBP «Ekonomika», 324. Available at: https://www.researchgate.net/publication/390199010_Resursi_strategicnih_korisnih_kopalin_Ukraini
- China hits back at US tariffs with export controls on key rare earths. Available at: https://www.reuters.com/world/china-hits-back-us-tariffs-with-rare-earth-export-controls-2025-04-04/
- Kotok, V., Kovalenko, V. (2018). A study of the effect of tungstate ions on the electrochromic properties of Ni(OH)2 films. Eastern-European Journal of Enterprise Technologies, 5 (12 (95)), 18–24. https://doi.org/10.15587/1729-4061.2018.145223
- Lv, L., Meng, J., Liu, C., Zhang, J., Wang, X., Bu, C. et al. (2026). Upcycling valuable metals of spent LIB cathode into high-performance catalysts for CO2-CH4 dry reforming. Fuel, 405, 136429. https://doi.org/10.1016/j.fuel.2025.136429
- Kovalenko, V., Kotok, V. (2019). Influence of the carbonate ion on characteristics of electrochemically synthesized layered (α+β) nickel hydroxide. Eastern-European Journal of Enterprise Technologies, 1 (6 (97)), 40–46. https://doi.org/10.15587/1729-4061.2019.155738
- Solovov, V. A., Nikolenko, N. V., Kovalenko, V. L., Kotok, V. A., Burkov, A. A., Kondrat'ev, D. A. et al. (2018). Synthesis of NI(II)-TI(IV) layered double hydroxides using coprecipitation at high supersaturation method. ARPN Journal of Engineering and Applied Sciences, 13 (24), 9652–9656. Available at: https://www.arpnjournals.org/jeas/research_papers/rp_2018/jeas_1218_7500.pdf
- Sihai, L., Xiangfan, N., Liucheng, Z., Xi, Y., Weifeng, H., Yinghong, L. (2017). Thermal stability of surface nanostructure produced by laser shock peening in a Ni-based superalloy. Surface and Coatings Technology, 311, 337–343. https://doi.org/10.1016/j.surfcoat.2017.01.031
- Zhang, Y., Fan, Y., Feng, K., Lu, C., Wang, Y., Shao, T. (2024). Evolution of high-temperature hardness of multimodal γ′ nickel-based superalloy. Journal of Materials Research and Technology, 29, 3771–3781. https://doi.org/10.1016/j.jmrt.2024.02.093
- Wolf, A. (2022). Stockpiling of Critical Metals as a Risk Management Strategy for Importing Countries. Journal of Resilient Economies (ISSN: 2653-1917), 2 (2). https://doi.org/10.25120/jre.2.2.2022.3931
- Kotok, V., Butyrina, T., Sknar, Y., Demchyshyna, O., Liashenko, A., Sukha, I. (2024). Determination of processing conditions for a heat-resistant superalloy used in turbine elements. Eastern-European Journal of Enterprise Technologies, 5 (12 (131)), 6–12. https://doi.org/10.15587/1729-4061.2024.313452
- Srivastava, R. R., Kim, M., Lee, J., Jha, M. K., Kim, B.-S. (2014). Resource recycling of superalloys and hydrometallurgical challenges. Journal of Materials Science, 49 (14), 4671–4686. https://doi.org/10.1007/s10853-014-8219-y
- Kovalenko, V., Kotok, V. (2020). Investigation of the anodic behavior of w-based superalloy for electrochemical selective treatment. Eastern-European Journal of Enterprise Technologies, 6 (12 (108)), 55–60. https://doi.org/10.15587/1729-4061.2020.218355
- Кovalenko, V., Kotok, V. (2017). Selective anodic treatment of W(WC)-based superalloy scrap. Eastern-European Journal of Enterprise Technologies, 1 (5 (85)), 53–58. https://doi.org/10.15587/1729-4061.2017.91205
- Kim, M.-S., Lee, J.-C., Park, H.-S., Jun, M.-J., Kim, B.-S. (2018). A multistep leaching of nickel-based superalloy scrap for selective dissolution of its constituent metals in hydrochloric acid solutions. Hydrometallurgy, 176, 235–242. https://doi.org/10.1016/j.hydromet.2018.02.002
- Mamo, S. K., Elie, M., Baron, M. G., Simons, A. M., Gonzalez-Rodriguez, J. (2019). Leaching kinetics, separation, and recovery of rhenium and component metals from CMSX-4 superalloys using hydrometallurgical processes. Separation and Purification Technology, 212, 150–160. https://doi.org/10.1016/j.seppur.2018.11.023
- Ali, I., Gaydukova, A., Kon’kova, T., ALOthman, Z. A., Sillanpää, M. (2023). Kinetics and Optimization of Metal Leaching from Heat-Resistant Nickel Alloy Solid Wastes. Molecules, 28 (14), 5545. https://doi.org/10.3390/molecules28145545
- Constantin, A. D., Hall, S., Pourhossein, F., Farnaud, S. (2025). Strategies for Nickel and Cobalt Mobilisation from Ni-Based Superalloy Residue Powders Using a Sustainable and Cost-Effective Bioleaching Method. Processes, 13 (7), 2157. https://doi.org/10.3390/pr13072157
- Wang, S., Sun, F., Liu, X., Chen, X., Li, J., He, L., Zhao, Z. (2025). A decomposition method for nickel-based superalloys by sulfurization: Recycling valuable metals. Journal of Alloys and Compounds, 1025, 180359. https://doi.org/10.1016/j.jallcom.2025.180359
- Tian, Q., Gan, X., Cui, F., Yu, D., Guo, X. (2021). Selective Extraction of Ni from Superalloy Scraps by Molten Mg-Zn. Metals, 11 (6), 993. https://doi.org/10.3390/met11060993
- Wang, L., Lu, S., Fan, J., Ma, Y., Zhang, J., Wang, S. et al. (2022). Recovery of Rare Metals from Superalloy Scraps by an Ultrasonic Leaching Method with a Two-Stage Separation Process. Separations, 9 (7), 184. https://doi.org/10.3390/separations9070184
- Ge, Y., Zhu, Z., Wang, D. (2017). Electrochemical Dissolution Behavior of the Nickel-Based Cast Superalloy K423A in NaNO3 Solution. Electrochimica Acta, 253, 379–389. https://doi.org/10.1016/j.electacta.2017.09.046
- Kovalenko, V., Kotok, V., Yeroshkina, A., Zaychuk, A. (2017). Synthesis and characterisation of dyeintercalated nickelaluminium layereddouble hydroxide as a cosmetic pigment. Eastern-European Journal of Enterprise Technologies, 5 (12 (89)), 27–33. https://doi.org/10.15587/1729-4061.2017.109814
- Kovalenko, V., Kotok, V., Kovalenko, I. (2018). Activation of the nickel foam as a current collector for application in supercapacitors. Eastern-European Journal of Enterprise Technologies, 3 (12 (93)), 56–62. https://doi.org/10.15587/1729-4061.2018.133472
- Kotok, V. A., Kovalenko, V. L. (2019). Non-Metallic Films Electroplating on the Low-Conductivity Substrates: The Conscious Selection of Conditions Using Ni(OH)2Deposition as an Example. Journal of The Electrochemical Society, 166 (10), D395–D408. https://doi.org/10.1149/2.0561910jes
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2025 Valerii Kotok, Yuri Sknar, Tatyana Butyrina, Irina Sknar, Irina Sukha, Oksana Demchyshyna

This work is licensed under a Creative Commons Attribution 4.0 International License.
The consolidation and conditions for the transfer of copyright (identification of authorship) is carried out in the License Agreement. In particular, the authors reserve the right to the authorship of their manuscript and transfer the first publication of this work to the journal under the terms of the Creative Commons CC BY license. At the same time, they have the right to conclude on their own additional agreements concerning the non-exclusive distribution of the work in the form in which it was published by this journal, but provided that the link to the first publication of the article in this journal is preserved.
A license agreement is a document in which the author warrants that he/she owns all copyright for the work (manuscript, article, etc.).
The authors, signing the License Agreement with TECHNOLOGY CENTER PC, have all rights to the further use of their work, provided that they link to our edition in which the work was published.
According to the terms of the License Agreement, the Publisher TECHNOLOGY CENTER PC does not take away your copyrights and receives permission from the authors to use and dissemination of the publication through the world's scientific resources (own electronic resources, scientometric databases, repositories, libraries, etc.).
In the absence of a signed License Agreement or in the absence of this agreement of identifiers allowing to identify the identity of the author, the editors have no right to work with the manuscript.
It is important to remember that there is another type of agreement between authors and publishers – when copyright is transferred from the authors to the publisher. In this case, the authors lose ownership of their work and may not use it in any way.








