Аналітичне дослідження кінетики модифікації неметалевих включень в процесі обробки розплаву сталі кальцієм
DOI:
https://doi.org/10.31498/2225-6733.49.1.2024.321262Ключові слова:
модифікація, неметалеві включення, термодинамічні розрахунки, гетерогенний процес, рівноважний стан, дифузіяАнотація
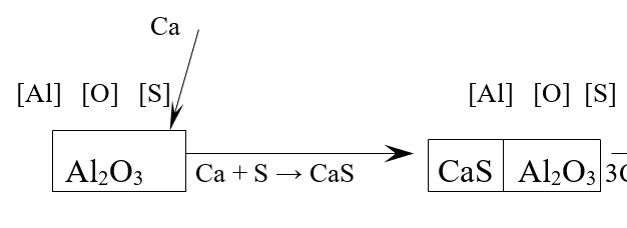
У цьому дослідженні показано, що сталі, які були розкислені алюмінієм та містять у своєму складі розчинену сірку, під час обробки кальцієм утворюють твердий сульфід кальцію, при цьому СаО буде присутній у вигляді алюмінатів кальцію, з відповідною зміною складу в залежності від температури. З’ясовано, що для кожної стадії реакцій перетворення алюмінатів кальцію рівноважні концентрації продуктів реакції будуть мати стале значення за умови сталості коефіцієнтів активності сірки та алюмінію відповідно. В дослідженні наведено зміну рівноважних продуктів реакції в результаті перебігу хімічного процесу та встановлено, що на останніх стадіях процесу підвищується активність СаО та знижується активність Al2O3. В процесі досліджень було встановлено кінетичну модель перебігу гетерогенного процесу модифікування розплаву сталі кальцієм. У наших дослідженнях встановлено, що механізм розчинення кальцію у рідкій сталі проходить крізь утворення проміжної сполуки. Визначено оптимальну концентрацію кальцію у межах 1 ppm, що буде інгібітором процесу утворення CaS-Al2O3. Показано, що лімітуючою стадією процесу буде транспортування Al та S від включень у об’єм рідкої сталі. В роботі показано, що для підтвердження аналітичного аналізу та перевірки стадії, що лімітує процес, а саме реакція CaS-Al2O3, за рахунок розчиненого Са у якості проміжної сполуки, полягає у розрахунку швидкості перетворення включень. Наведено, що концентрації розчинених алюмінію та сірки, що перебувають у рівновазі з включеннями, дуже малі, а загальний час, що необхідний для модифікації включень, у багато разів перевищував рівноважні концентрації. Для врахування цих умов нами було створено кінетичну модель для оцінки часу, що дозволила визначити стадію, що лімітує швидкість загального процесу модифікування включень. Для визначення і розрахунків рівноважних умов співіснування двох фаз у наших дослідженнях було використано термодинамічний пакет програм FactSage 8.3. З використанням термодинамічних розрахунків встановлено час, необхідний для перетворення включень глинозема у основні рідкі оксидні включення. В дослідженнях показано, що реакція модифікації включень напряму залежить від ступеня витрати твердого CaS. Для кожної стадії реакції перетворення включень швидкість має стале значення, що підтверджується сталою швидкістю видалення CaS. В результаті проведених досліджень та термодинамічних розрахунків встановлено, що гетерогенний процес модифікації включень перебігає у дифузійній області і контролюється масопереносом. Час модифікації лінійно зростає зі зростанням вмісту кисню та зменшується зі зростанням вмісту кальцію у розплаві сталі. Доведено, що на заключній стадії модифікації включень необхідно в шість разів більше часу, ніж на ранніх стадіях процесу модифікування
Посилання
Xi Z., Li C., Wang L. A kinetic model for the modification of Al2O3 inclusions during calcium treatment in high-carbon hard wire steel. Materials. 2021. Vol. 14(5), Pp. 2-16. DOI: https://doi.org/10.3390/ma14051305.
Li X. D., Deng S., Yang Y. B. Production practice of plasticity control of inclusions in CaO–Al2O3–SiO2 system of SWRH82B hard wire steel. Metall. China. 2018. Vol. 28. Pp. 61-66.
Behaviour of Sulphide and Non-alumina-Based Oxide Inclusions in Ca-Treated High-Carbon Steel / Y. Tanaka et al. Metallurgical and Matersals Transaction A. 2020. Vol. 51. Pp. 1384-1394. DOI: https://doi.org/10.1007/s11663-020-01872-2.
Handoko W., Anurag A., Pahlevani F. Effect of selective-precipitations process on the corrosion resistance and hardness of dual-phase high-carbon steel. Scientific reports. 2019. Vol. 9. Pp. 1-16. DOI: https://doi.org/10.1038/s41598-019-52228-z.
Wang G. D., Lv Y. W. Research on inclusion control and rolling process optimization of 82B hard wire steel. Metall. China. 2012. Vol. 22. Pp. 15-17.
Finite element simulation of cold rolling of thin strip / Z. Jiang et al. Journal of Materials Processing Technology. 2003. Vol. 140. Pp. 542-547. DOI: https://doi.org/10.1016/S0924-0136(03)00832-X.
Abraham S., Bodnar R., Raines J. Inclusion engineering and metallurgy of calcium treatment. Journal of Iron and Steel Research International. 2018. Vol. 1. Pp. 1243-1257. DOI: http://dx.doi.org/10.1007/s42243-018-0017-3.
Transient Inclusion Evolution During Modification of Alumina Inclusions by Calcium in Liquid Steel: Part I. Background, Experimental Techniques and Analysis Methods / N. Verma et al. Metallurgical and Materials Transaction B. 2011. Vol. 42. Pp. 711-719. DOI: http://dx.doi.org/10.1007/s11663-011-9517-2.
Yang G. W., Wang X. H., Huang F. X. Influence of Calcium Addition on Inclusions in LCAK Steel with Ultralow Sulfur Content. Metallurgical and Materials Transaction B. 2015. Vol. 46. Pp. 145-154. DOI: http://dx.doi.org/10.1007/s11663-014-0181-1.
Zhang L., Liu Y., Zhang Y. Transient Evolution of Nonmetallic Inclusions During Calcium Treatment of Molten Steel. Metallurgical and Materials Transaction B. 2018. Vol. 49. Pp. 1-19. DOI: https://doi.org/10.1007/s11663-018-1289-5.
Sa´nchez G., Hidalgo C., Donoso P. Kinetic studies of calcium-Induced calcium release in cardiac sarcoplasmic reticulum vesicles. Biophysical Journal. 2003. Vol. 84. Pp. 2319–2330. https://doi.org/10.1016/S0006-3495(03)75037-1.
##submission.downloads##
Опубліковано
Як цитувати
Номер
Розділ
Ліцензія

Ця робота ліцензується відповідно до Creative Commons Attribution 4.0 International License.
Журнал "Вісник Приазовського державного технічного університету. Серія: Технічні науки" видається під ліцензією СС-BY (Ліцензія «Із зазначенням авторства»).
Дана ліцензія дозволяє поширювати, редагувати, поправляти і брати твір за основу для похідних навіть на комерційній основі із зазначенням авторства. Це найзручніша з усіх пропонованих ліцензій. Рекомендується для максимального поширення і використання неліцензійних матеріалів.
Автори, які публікуються в цьому журналі, погоджуються з наступними умовами:
1. Автори залишають за собою право на авторство своєї роботи та передають журналу право першої публікації цієї роботи на умовах ліцензії Creative Commons Attribution License, яка дозволяє іншим особам вільно розповсюджувати опубліковану роботу з обов'язковим посиланням на авторів оригінальної роботи та першу публікацію роботи в цьому журналі.
2. Автори мають право укладати самостійні додаткові угоди, які стосуються неексклюзивного поширення роботи в тому вигляді, в якому вона була опублікована цим журналом (наприклад, розміщувати роботу в електронному сховищі установи або публікувати у складі монографії), за умови збереження посилання на першу публікацію роботи в цьому журналі.








