The effect of coating concentration of curcumin: H2O on copper winding characteristics
DOI:
https://doi.org/10.15587/1729-4061.2023.275727Keywords:
aromatic ring, electron spin, magnetic field, copper coil, curcumin concentrationAbstract
Each coil of copper produces a magnetic field and the total field inside the solenoid will be the sum of the fields caused by each coil of current. If the solenoid coils are very closely spaced, the internal field will be essentially parallel to the axis except at the very ends. To find out the magnitude of the magnetic field inside the solenoid, you can use Ampere’s law, namely B=μo∙N∙I, where B is the magnetic field strength (T), µo is air permeability (4×10‒7 T m/A), N is the number of turns and I is an electric current. The value of B depends on the number of turns per unit length, N, and current I. The field is independent of the position inside the solenoid, so the value of B is uniform. This only applies to infinite solenoids, but is a good approximation for actual points that are not near the ends of the solenoid.
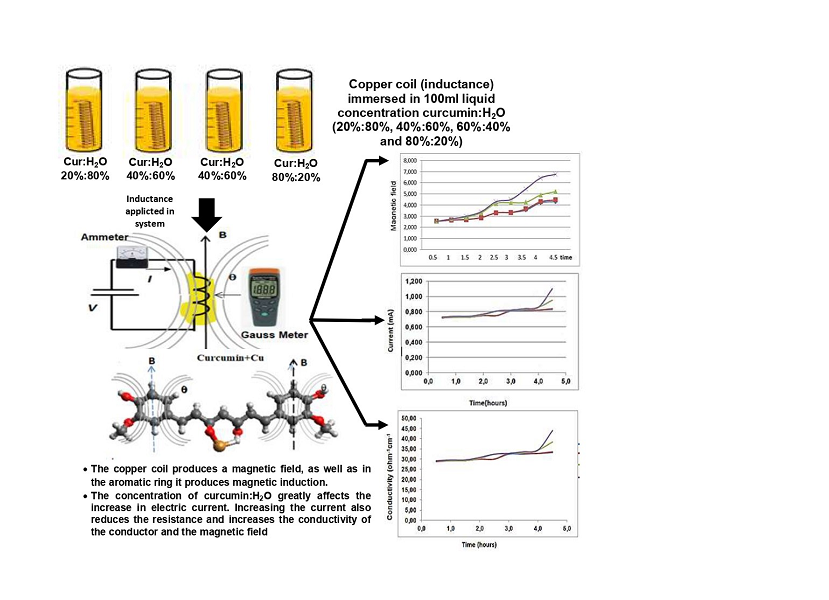
The research object is 4 identical copper coils with a length of 3 cm, a coil diameter of 2 cm, a cross section of 1.5 mm2 with an inductance value of 2.17 µH. Before coating curcumin on the copper winding, the initial value of the magnetic field strength was 2.54 µTesla. After the coating process of curcumin:H2O concentration, the value of the magnetic field strength increased.
The method used was immersing 4 copper coils with an inductance value of 2.17 µH in curcumin:H2O concentration in a 100 ml volume measuring cup, with the respective concentrations: (20 %:80 %), (40 %:60 %), (60 %:40 %), (80 %:20 %) in a certain time. Then the copper coil conductor is supplied with a 5-volt DC voltage source. Then the value of the magnetic field strength (B) and electric current is measured, the results are compared with the system before immersing the copper coil.
The measurement results showed that the values of electric current and magnetic field strength increased after curcumin coating compared to before treatment. To see the bonding performance of curcumin and copper, the FTIR test and simulation of the curcumin: copper bond were carried out using Avogadro software. In the IR test, there is a strong absorption of aromatic C-C from 1,650 cm-1 to 1,500 cm-1. Whereas in the simulation, the bond between copper and curcumin produces a bond energy of 164.532 kJ/mol or equivalent to 171.12×10-2 eV
Supporting Agency
- Universitas Brawijaya Malang
References
- Revathy, S., Elumalai, S., Benny, M., Antony, B. (2011). Isolation, Purification and Identification of Curcuminoids from Turmeric (Curcuma longa L.) by Column Chromatography. Journal of Experimental Sciences, 2 (7), 21–25.
- Mary, C. P. V., Vijayakumar, S., Shankar, R. (2018). Metal chelating ability and antioxidant properties of Curcumin-metal complexes – A DFT approach. Journal of Molecular Graphics and Modelling, 79, 1–14. doi: https://doi.org/10.1016/j.jmgm.2017.10.022
- Abdul Zahar, Z., Mohsin, H. F., Ibtisam, A. (2020). The Study on Curcuminoids in Chromatography, Spectroscopy and Regioisomerism. Journal of Physics: Conference Series, 1529 (2), 022035. doi: https://doi.org/10.1088/1742-6596/1529/2/022035
- Okonogi, S., Naksuriya, O., Charumanee, S., Sirithunyalug, J. (2016). Effect of Aromatic Substitution of Curcumin Nanoformulations on Their Stability. Scientia Pharmaceutica, 84 (4), 625–633. doi: https://doi.org/10.3390/scipharm84040625
- Nelson, K. M., Dahlin, J. L., Bisson, J., Graham, J., Pauli, G. F., Walters, M. A. (2017). The Essential Medicinal Chemistry of Curcumin. Journal of Medicinal Chemistry, 60 (5), 1620–1637. doi: https://doi.org/10.1021/acs.jmedchem.6b00975
- Agne, E. B. P., Hastuti, R., Khabibi, K. (2010). Ekstraksi dan Uji Kestabilan Zat Warna Betasianin dari Kulit Buah Naga (Hylocereus polyrhizus) serta Aplikasinya sebagai Pewarna Alami Pangan. Jurnal Kimia Sains Dan Aplikasi, 13 (2), 51–56. doi: https://doi.org/10.14710/jksa.13.2.51-56
- Barik, A., Mishra, B., Kunwar, A., Kadam, R. M., Shen, L., Dutta, S. et al. (2007). Comparative study of copper(II)–curcumin complexes as superoxide dismutase mimics and free radical scavengers. European Journal of Medicinal Chemistry, 42 (4), 431–439. doi: https://doi.org/10.1016/j.ejmech.2006.11.012
- Bergman, J. (2022). Metal Properties: Conductivity. Available at: https://blog.eaglegroupmanufacturers.com/metal-properties-conductivity
- About Conductivity. Available at: https://www.lehigh.edu/~amb4/wbi/kwardlow/conductivity.htm
- Bowler, N., Huang, Y. (2005). Electrical conductivity measurement of metal plates using broadband eddy-current and four-point methods. Measurement Science and Technology, 16 (11), 2193–2200. doi: https://doi.org/10.1088/0957-0233/16/11/009
- Meza-Morales, W., Estévez-Carmona, M. M., Alvarez-Ricardo, Y., Obregón-Mendoza, M. A., Cassani, J., Ramírez-Apan, M. T. et al. (2019). Full Structural Characterization of Homoleptic Complexes of Diacetylcurcumin with Mg, Zn, Cu, and Mn: Cisplatin-level Cytotoxicity in Vitro with Minimal Acute Toxicity in Vivo. Molecules, 24 (8), 1598. doi: https://doi.org/10.3390/molecules24081598
- Morales, N. P., Sirijaroonwong, S., Yamanont, P., Phisalaphong, C. (2015). Electron Paramagnetic Resonance Study of the Free Radical Scavenging Capacity of Curcumin and Its Demethoxy and Hydrogenated Derivatives. Biological and Pharmaceutical Bulletin, 38 (10), 1478–1483. doi: https://doi.org/10.1248/bpb.b15-00209
- Khorasani, M. Y., Langari, H., Sany, S. B. T., Rezayi, M., Sahebkar, A. (2019). The role of curcumin and its derivatives in sensory applications. Materials Science and Engineering: C, 103, 109792. doi: https://doi.org/10.1016/j.msec.2019.109792
- Lustiani, D. (2009). Syntesis of Curcumin analogues 3, 6-Bis-(4-Hidroksi-3-Metoksibenzilidin)-Piperazin-2.5-Dion with Catalyst HCl.
- Radi, A.-E., El-Ghany, N. A., Wahdan, T. (2016). Determination of Esomeprazole on an Electropolymerized L-arginine and β-cyclodextrin Modified Screen Printed Carbon Electrode. Electroanalysis, 28 (5), 1112–1118. doi: https://doi.org/10.1002/elan.201501074
- Furukawa, S., Fujita, M., Kanatomi, Y., Minoura, M., Hatanaka, M., Morokuma, K. et al. (2018). Double aromaticity arising from σ- and π-rings. Communications Chemistry, 1 (1). doi: https://doi.org/10.1038/s42004-018-0057-4
- Zhao, H.-L. (2017). Quantum mechanical calculation of electron spin. Open Physics, 15 (1), 652–661. doi: https://doi.org/10.1515/phys-2017-0076
- Particle on a Ring. Available at: https://chem.libretexts.org/Bookshelves/Physical_and_Theoretical_Chemistry_Textbook_Maps/Supplemental_Modules_(Physical_and_Theoretical_Chemistry)/Quantum_Mechanics/05.5%3A_Particle_in_Boxes/Particle_on_a_Ring
- Satrio, W., Winarto, Sugiono, Wardana, I. N. G. (2020). The effect of curcumin coated electrode on hydrogen production through water electrolysis. E3S Web of Conferences, 181, 01003. doi: https://doi.org/10.1051/e3sconf/202018101003
- Rauhalahti, M. (2022). Quantum Chemical Studies of Ring Currents of Aromatic Molecules. Helsinki. Available at: https://helda.helsinki.fi/bitstream/handle/10138/350473/Rauhalahti_Markus_dissertation_2022.pdf?sequence=1
- Electrophilic Aromatic Substitution – The Mechanism. Available at: https://www.masterorganicchemistry.com/2017/11/09/electrophilic-aromatic-substitution-the-mechanism/
- Benzene and Aromaticity. Available at: https://chem.libretexts.org/Bookshelves/Physical_and_Theoretical_Chemistry_Textbook_Maps/Physical_Chemistry_(LibreTexts)/10%3A_Bonding_in_Polyatomic_Molecules/10.07%3A_Benzene_and_Aromaticity
- Çakır, S., Biçer, E., Yılmaz Arslan, E. (2015). A Newly Developed Electrocatalytic Oxidation and Voltammetric Determination of Curcumin at the Surface of PdNp-graphite Electrode by an Aqueous Solution Process with Al3+. Croatica Chemica Acta, 88 (2), 105–112. doi: https://doi.org/10.5562/cca2527
- Hu, L., Shi, D., Li, X., Zhu, J., Mao, F. et al. (2020). Curcumin-based polarity fluorescent probes: Design strategy and biological applications. Dyes and Pigments, 177, 108320. doi: https://doi.org/10.1016/j.dyepig.2020.108320
- Merino, G., Heine, T., Seifert, G. (2004). The Induced Magnetic Field in Cyclic Molecules. Chemistry - A European Journal, 10 (17), 4367–4371. doi: https://doi.org/10.1002/chem.200400457
- University Physics. Available at: https://phys.libretexts.org/Bookshelves/University_Physics
- Kanno, M., Hoki, K., Kono, H., Fujimura, Y. (2007). Quantum optimal control of electron ring currents in chiral aromatic molecules. The Journal of Chemical Physics, 127 (20), 204314. doi: https://doi.org/10.1063/1.2806180
- Mineo, H., Phan, N.-L., Fujimura, Y. (2021). Quantum Control of Coherent π-Electron Dynamics in Aromatic Ring Molecules. Frontiers in Physics, 9. doi: https://doi.org/10.3389/fphy.2021.675134
- Jirásek, M., Anderson, H. L., Peeks, M. D. (2021). From Macrocycles to Quantum Rings: Does Aromaticity Have a Size Limit? Accounts of Chemical Research, 54 (16), 3241–3251. doi: https://doi.org/10.1021/acs.accounts.1c00323
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2023 Zainal Abidin, Eko Siswanto, Widya Wijayanti, Winarto

This work is licensed under a Creative Commons Attribution 4.0 International License.
The consolidation and conditions for the transfer of copyright (identification of authorship) is carried out in the License Agreement. In particular, the authors reserve the right to the authorship of their manuscript and transfer the first publication of this work to the journal under the terms of the Creative Commons CC BY license. At the same time, they have the right to conclude on their own additional agreements concerning the non-exclusive distribution of the work in the form in which it was published by this journal, but provided that the link to the first publication of the article in this journal is preserved.
A license agreement is a document in which the author warrants that he/she owns all copyright for the work (manuscript, article, etc.).
The authors, signing the License Agreement with TECHNOLOGY CENTER PC, have all rights to the further use of their work, provided that they link to our edition in which the work was published.
According to the terms of the License Agreement, the Publisher TECHNOLOGY CENTER PC does not take away your copyrights and receives permission from the authors to use and dissemination of the publication through the world's scientific resources (own electronic resources, scientometric databases, repositories, libraries, etc.).
In the absence of a signed License Agreement or in the absence of this agreement of identifiers allowing to identify the identity of the author, the editors have no right to work with the manuscript.
It is important to remember that there is another type of agreement between authors and publishers – when copyright is transferred from the authors to the publisher. In this case, the authors lose ownership of their work and may not use it in any way.








