Вплив концентрації покриття куркумін: H2O на характеристики мідної обмотки
DOI:
https://doi.org/10.15587/1729-4061.2023.275727Ключові слова:
ароматичне кільце, спін електрона, магнітне поле, мідна котушка, концентрація куркумінуАнотація
Кожна мідна котушка створює магнітне поле, і загальне поле всередині соленоїда дорівнюватиме сумі полів, що утворюються кожною котушкою струму. Якщо соленоїдні котушки розташовані дуже близько одна до одної, внутрішнє поле буде по суті паралельним осі, за винятком самих кінців. Для визначення величини магнітного поля всередині соленоїда можна скористатися законом Ампера, а саме B=μo∙N∙I, де B ‒ напруженість магнітного поля (Тл), μo ‒ повітропроникність (4×10-7 Тл м/А), N ‒ кількість витків, I ‒ електричний струм. Значення B залежить від кількості витків на одиницю довжини, N і струму I. Поле не залежить від положення всередині соленоїда, тому значення B є постійним. Це стосується лише нескінченних соленоїдів, але є хорошим наближенням для фактичних точок, які не знаходяться поблизу кінців соленоїда.
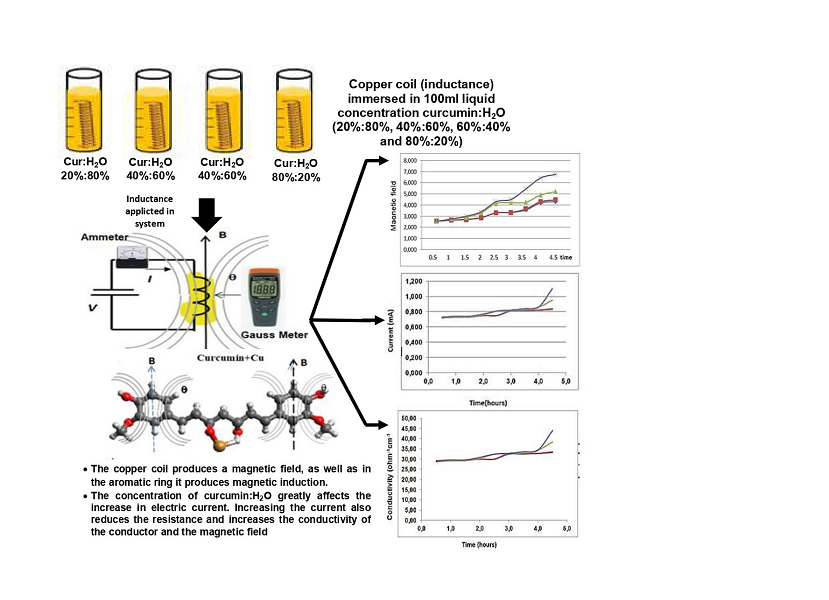
Об'єктом дослідження є 4 однакові мідні котушки довжиною 3 см, діаметром котушки 2 см, перерізом1,5 мм2 та величиною індуктивності 2,17 мкГн. Перед нанесенням куркуміну на мідну обмотку початкове значення напруженості магнітного поля становило 2,54 мкТл. Після процесу покриття концентрації куркуміну:H2O значення напруженості магнітного поля збільшилося.
Використовуваний метод полягав у зануренні 4 мідних котушок зі значенням індуктивності 2,17 мкГн у концентрацію куркумін:H2O у мірній склянці об'ємом 100 мл з відповідними концентраціями: (20 %:80 %), (40 %:60 %), (60 %:40 %), (80 %:20 %) протягом певного часу. Потім на провідник мідної котушки подається 5-вольтове джерело постійної напруги. Далі вимірюється значення напруженості магнітного поля (B) та електричного струму, результати порівнюються з системою перед зануренням мідної котушки.
Результати вимірювань показали збільшення значень електричного струму та напруженості магнітного поля після покриття куркуміном порівняно зі значеннями до обробки. Для оцінки ефективності зв'язування куркуміну та міді було проведено FTIR-випробування та моделювання зв'язку куркумін: мідь за допомогою програмного забезпечення Avogadro. При ІЧ-випробуванні спостерігається сильне поглинання ароматичного C-C у межах від 1650 см-1 до 1500 см-1. Тоді як при моделюванні зв'язок між міддю та куркуміном дає енергію зв'язку 164,532 кДж/моль, що еквівалентно 171,12×10-2 еВ
Спонсор дослідження
- Universitas Brawijaya Malang
Посилання
- Revathy, S., Elumalai, S., Benny, M., Antony, B. (2011). Isolation, Purification and Identification of Curcuminoids from Turmeric (Curcuma longa L.) by Column Chromatography. Journal of Experimental Sciences, 2 (7), 21–25.
- Mary, C. P. V., Vijayakumar, S., Shankar, R. (2018). Metal chelating ability and antioxidant properties of Curcumin-metal complexes – A DFT approach. Journal of Molecular Graphics and Modelling, 79, 1–14. doi: https://doi.org/10.1016/j.jmgm.2017.10.022
- Abdul Zahar, Z., Mohsin, H. F., Ibtisam, A. (2020). The Study on Curcuminoids in Chromatography, Spectroscopy and Regioisomerism. Journal of Physics: Conference Series, 1529 (2), 022035. doi: https://doi.org/10.1088/1742-6596/1529/2/022035
- Okonogi, S., Naksuriya, O., Charumanee, S., Sirithunyalug, J. (2016). Effect of Aromatic Substitution of Curcumin Nanoformulations on Their Stability. Scientia Pharmaceutica, 84 (4), 625–633. doi: https://doi.org/10.3390/scipharm84040625
- Nelson, K. M., Dahlin, J. L., Bisson, J., Graham, J., Pauli, G. F., Walters, M. A. (2017). The Essential Medicinal Chemistry of Curcumin. Journal of Medicinal Chemistry, 60 (5), 1620–1637. doi: https://doi.org/10.1021/acs.jmedchem.6b00975
- Agne, E. B. P., Hastuti, R., Khabibi, K. (2010). Ekstraksi dan Uji Kestabilan Zat Warna Betasianin dari Kulit Buah Naga (Hylocereus polyrhizus) serta Aplikasinya sebagai Pewarna Alami Pangan. Jurnal Kimia Sains Dan Aplikasi, 13 (2), 51–56. doi: https://doi.org/10.14710/jksa.13.2.51-56
- Barik, A., Mishra, B., Kunwar, A., Kadam, R. M., Shen, L., Dutta, S. et al. (2007). Comparative study of copper(II)–curcumin complexes as superoxide dismutase mimics and free radical scavengers. European Journal of Medicinal Chemistry, 42 (4), 431–439. doi: https://doi.org/10.1016/j.ejmech.2006.11.012
- Bergman, J. (2022). Metal Properties: Conductivity. Available at: https://blog.eaglegroupmanufacturers.com/metal-properties-conductivity
- About Conductivity. Available at: https://www.lehigh.edu/~amb4/wbi/kwardlow/conductivity.htm
- Bowler, N., Huang, Y. (2005). Electrical conductivity measurement of metal plates using broadband eddy-current and four-point methods. Measurement Science and Technology, 16 (11), 2193–2200. doi: https://doi.org/10.1088/0957-0233/16/11/009
- Meza-Morales, W., Estévez-Carmona, M. M., Alvarez-Ricardo, Y., Obregón-Mendoza, M. A., Cassani, J., Ramírez-Apan, M. T. et al. (2019). Full Structural Characterization of Homoleptic Complexes of Diacetylcurcumin with Mg, Zn, Cu, and Mn: Cisplatin-level Cytotoxicity in Vitro with Minimal Acute Toxicity in Vivo. Molecules, 24 (8), 1598. doi: https://doi.org/10.3390/molecules24081598
- Morales, N. P., Sirijaroonwong, S., Yamanont, P., Phisalaphong, C. (2015). Electron Paramagnetic Resonance Study of the Free Radical Scavenging Capacity of Curcumin and Its Demethoxy and Hydrogenated Derivatives. Biological and Pharmaceutical Bulletin, 38 (10), 1478–1483. doi: https://doi.org/10.1248/bpb.b15-00209
- Khorasani, M. Y., Langari, H., Sany, S. B. T., Rezayi, M., Sahebkar, A. (2019). The role of curcumin and its derivatives in sensory applications. Materials Science and Engineering: C, 103, 109792. doi: https://doi.org/10.1016/j.msec.2019.109792
- Lustiani, D. (2009). Syntesis of Curcumin analogues 3, 6-Bis-(4-Hidroksi-3-Metoksibenzilidin)-Piperazin-2.5-Dion with Catalyst HCl.
- Radi, A.-E., El-Ghany, N. A., Wahdan, T. (2016). Determination of Esomeprazole on an Electropolymerized L-arginine and β-cyclodextrin Modified Screen Printed Carbon Electrode. Electroanalysis, 28 (5), 1112–1118. doi: https://doi.org/10.1002/elan.201501074
- Furukawa, S., Fujita, M., Kanatomi, Y., Minoura, M., Hatanaka, M., Morokuma, K. et al. (2018). Double aromaticity arising from σ- and π-rings. Communications Chemistry, 1 (1). doi: https://doi.org/10.1038/s42004-018-0057-4
- Zhao, H.-L. (2017). Quantum mechanical calculation of electron spin. Open Physics, 15 (1), 652–661. doi: https://doi.org/10.1515/phys-2017-0076
- Particle on a Ring. Available at: https://chem.libretexts.org/Bookshelves/Physical_and_Theoretical_Chemistry_Textbook_Maps/Supplemental_Modules_(Physical_and_Theoretical_Chemistry)/Quantum_Mechanics/05.5%3A_Particle_in_Boxes/Particle_on_a_Ring
- Satrio, W., Winarto, Sugiono, Wardana, I. N. G. (2020). The effect of curcumin coated electrode on hydrogen production through water electrolysis. E3S Web of Conferences, 181, 01003. doi: https://doi.org/10.1051/e3sconf/202018101003
- Rauhalahti, M. (2022). Quantum Chemical Studies of Ring Currents of Aromatic Molecules. Helsinki. Available at: https://helda.helsinki.fi/bitstream/handle/10138/350473/Rauhalahti_Markus_dissertation_2022.pdf?sequence=1
- Electrophilic Aromatic Substitution – The Mechanism. Available at: https://www.masterorganicchemistry.com/2017/11/09/electrophilic-aromatic-substitution-the-mechanism/
- Benzene and Aromaticity. Available at: https://chem.libretexts.org/Bookshelves/Physical_and_Theoretical_Chemistry_Textbook_Maps/Physical_Chemistry_(LibreTexts)/10%3A_Bonding_in_Polyatomic_Molecules/10.07%3A_Benzene_and_Aromaticity
- Çakır, S., Biçer, E., Yılmaz Arslan, E. (2015). A Newly Developed Electrocatalytic Oxidation and Voltammetric Determination of Curcumin at the Surface of PdNp-graphite Electrode by an Aqueous Solution Process with Al3+. Croatica Chemica Acta, 88 (2), 105–112. doi: https://doi.org/10.5562/cca2527
- Hu, L., Shi, D., Li, X., Zhu, J., Mao, F. et al. (2020). Curcumin-based polarity fluorescent probes: Design strategy and biological applications. Dyes and Pigments, 177, 108320. doi: https://doi.org/10.1016/j.dyepig.2020.108320
- Merino, G., Heine, T., Seifert, G. (2004). The Induced Magnetic Field in Cyclic Molecules. Chemistry - A European Journal, 10 (17), 4367–4371. doi: https://doi.org/10.1002/chem.200400457
- University Physics. Available at: https://phys.libretexts.org/Bookshelves/University_Physics
- Kanno, M., Hoki, K., Kono, H., Fujimura, Y. (2007). Quantum optimal control of electron ring currents in chiral aromatic molecules. The Journal of Chemical Physics, 127 (20), 204314. doi: https://doi.org/10.1063/1.2806180
- Mineo, H., Phan, N.-L., Fujimura, Y. (2021). Quantum Control of Coherent π-Electron Dynamics in Aromatic Ring Molecules. Frontiers in Physics, 9. doi: https://doi.org/10.3389/fphy.2021.675134
- Jirásek, M., Anderson, H. L., Peeks, M. D. (2021). From Macrocycles to Quantum Rings: Does Aromaticity Have a Size Limit? Accounts of Chemical Research, 54 (16), 3241–3251. doi: https://doi.org/10.1021/acs.accounts.1c00323
##submission.downloads##
Опубліковано
Як цитувати
Номер
Розділ
Ліцензія
Авторське право (c) 2023 Zainal Abidin, Eko Siswanto, Widya Wijayanti, Winarto

Ця робота ліцензується відповідно до Creative Commons Attribution 4.0 International License.
Закріплення та умови передачі авторських прав (ідентифікація авторства) здійснюється у Ліцензійному договорі. Зокрема, автори залишають за собою право на авторство свого рукопису та передають журналу право першої публікації цієї роботи на умовах ліцензії Creative Commons CC BY. При цьому вони мають право укладати самостійно додаткові угоди, що стосуються неексклюзивного поширення роботи у тому вигляді, в якому вона була опублікована цим журналом, але за умови збереження посилання на першу публікацію статті в цьому журналі.
Ліцензійний договір – це документ, в якому автор гарантує, що володіє усіма авторськими правами на твір (рукопис, статтю, тощо).
Автори, підписуючи Ліцензійний договір з ПП «ТЕХНОЛОГІЧНИЙ ЦЕНТР», мають усі права на подальше використання свого твору за умови посилання на наше видання, в якому твір опублікований. Відповідно до умов Ліцензійного договору, Видавець ПП «ТЕХНОЛОГІЧНИЙ ЦЕНТР» не забирає ваші авторські права та отримує від авторів дозвіл на використання та розповсюдження публікації через світові наукові ресурси (власні електронні ресурси, наукометричні бази даних, репозитарії, бібліотеки тощо).
За відсутності підписаного Ліцензійного договору або за відсутністю вказаних в цьому договорі ідентифікаторів, що дають змогу ідентифікувати особу автора, редакція не має права працювати з рукописом.
Важливо пам’ятати, що існує і інший тип угоди між авторами та видавцями – коли авторські права передаються від авторів до видавця. В такому разі автори втрачають права власності на свій твір та не можуть його використовувати в будь-який спосіб.









