Development of a preliminary model for addressing naphthenic acid corrosion of ASTM A-335 P9 using an eco-enzyme green inhibitor on heavy-vacuum gas oil residue
DOI:
https://doi.org/10.15587/1729-4061.2025.347916Keywords:
green corrosion inhibitor, biomass, eco-enzyme, Langmuir isotherm, adsorption, refineryAbstract
ASTM A-335 P9 is widely employed within the piping infrastructure of the crude distillation unit for the conveyance of heavy vacuum gas oil (HVGO) crude residue before subsequent downstream processing, owing to its high mechanical properties.
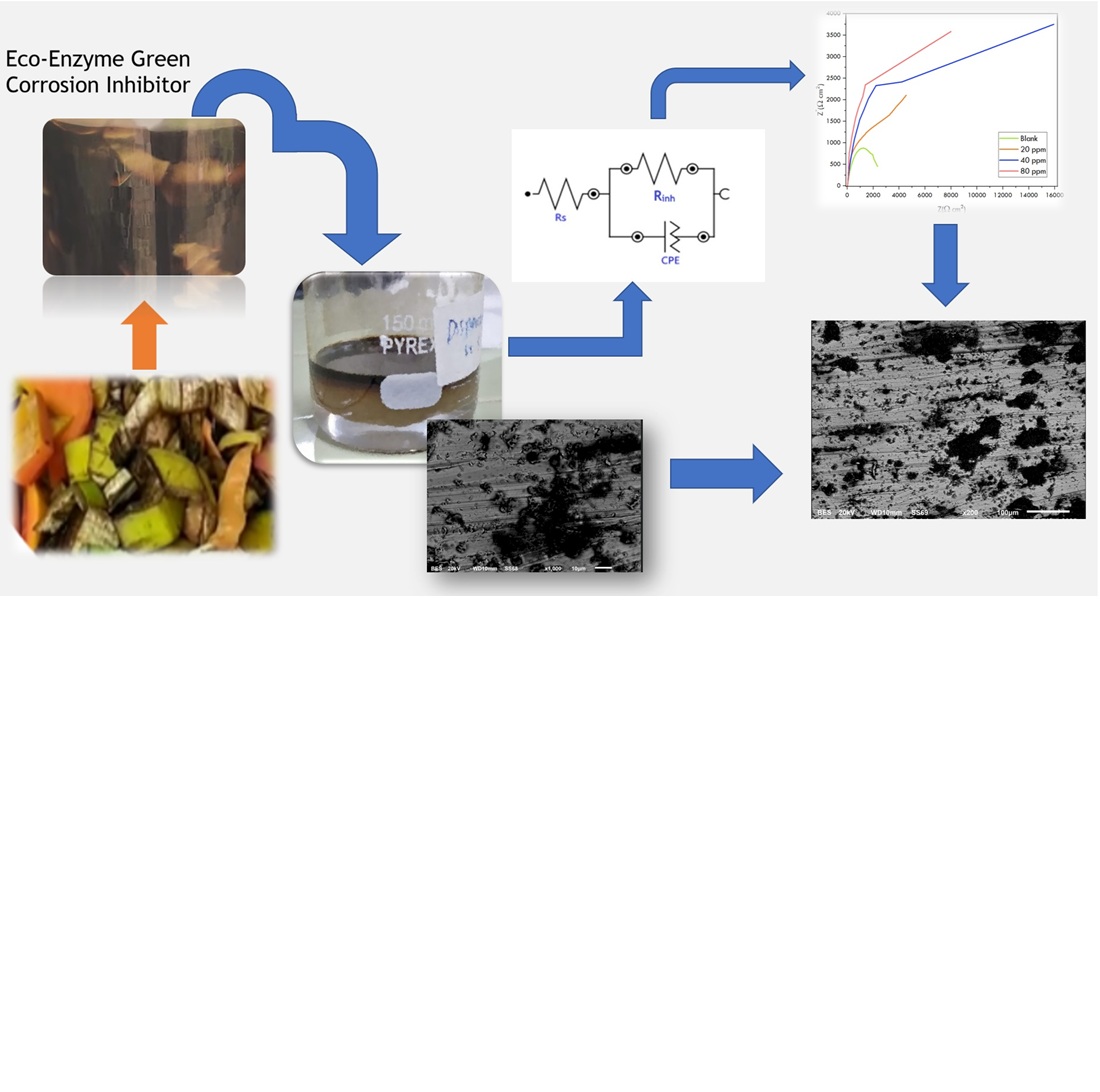
Due to elevated operating temperatures, the presence of sulfur, the material remains vulnerable to naphthenic acid corrosion, which can compromise its structural and operational integrity. This study examines the material's response to the application of eco-enzyme as a green corrosion inhibitor (GCI) employing extensive several tests under naphthenic acid distillate which collected and processed from the heavy vacuum gas oil piping. optical emission spectroscopy (OES), ultraviolet-visible spectroscopy (UV-Vis), and Fourier rransform infra-red (FTIR) equipped with potentiodynamic polarization and electrochemical impedance spectroscopy (EIS) to dive into the corrosion resistance of EE inhibitor under the naphthenic acid extracts. Moreover, the scanning electronic microscopy and energy X-Ray dispersive (SEM-EDX) was utilized to reveal the surface morphology and the elemental identity of the retained inhibition mechanism. Based on the OES, the presence of Cr and Mo are highlighted with the composition of 9.135% and 0.894% which aligned with the 9Cr-1Mo material specification. The electronic transition of π-π* and n-π* transitions is in good agreement with the presence of aromatic -OH, C-H sp3, R-CHO, C=O, C-O and aromatic absorption at 525 nm, which correlates with peaks observed in FTIR spectra at 3200–3400, 2800–3000 cm-1. The high inhibition efficiency beyond 77% is correlated to the adsorption of the inhibitor that thermodynamically adheres to the Langmuir adsorption isotherm and applicable of model the HVGO system
References
- Rodríguez-Antón, L. M., Gutiérrez-Martín, F., Martinez-Arevalo, C. (2015). Experimental determination of some physical properties of gasoline, ethanol and ETBE ternary blends. Fuel, 156, 81–86. https://doi.org/10.1016/j.fuel.2015.04.040
- Esouilem, M., Bouzid, A.-H., Nadeau, S. (2022). Pressure Vessels and Piping Accident Analysis and Prevention: A Case Study in Canada. International Journal of Safety and Security Engineering, 12 (1), 105–114. https://doi.org/10.18280/ijsse.120113
- Stepanova, N. E. (2022). Environmental safety and environmental protection at an oil refinery. Advances in Current Natural Sciences, 5, 78–83. https://doi.org/10.17513/use.37828
- Rajaram, K., Jaikumar, R., Behlau, F., van Esch, F., Heynen, C., Kaiser, R. et al. (1999). Robust Process Control at Cerestar’s Refineries. Interfaces, 29 (1), 30–48. https://doi.org/10.1287/inte.29.1.30
- Sukcharoen, K., Leatham, D. J. (2017). Hedging downside risk of oil refineries: A vine copula approach. Energy Economics, 66, 493–507. https://doi.org/10.1016/j.eneco.2017.07.012
- Verma, C., Ebenso, E. E., Quraishi, M. A. (2017). Corrosion inhibitors for ferrous and non-ferrous metals and alloys in ionic sodium chloride solutions: A review. Journal of Molecular Liquids, 248, 927–942. https://doi.org/10.1016/j.molliq.2017.10.094
- Siler-Evans, K., Hanson, A., Sunday, C., Leonard, N., Tumminello, M. (2014). Analysis of pipeline accidents in the United States from 1968 to 2009. International Journal of Critical Infrastructure Protection, 7 (4), 257–269. https://doi.org/10.1016/j.ijcip.2014.09.002
- Matthews, C. (2023). API RP 571: Damage Mechanisms (API 653). The API ICP Exam Handbook: Complete Guide to Passing the API 510/570/653 ICP Exams, 792–800. https://doi.org/10.1115/1.862api_ch38
- Qu, D., Zheng, Y., Jiang, X., Ke, W. (2007). Correlation between the corrosivity of naphthenic acids and their chemical structures. Anti-Corrosion Methods and Materials, 54 (4), 211–218. https://doi.org/10.1108/00035590710762348
- Chakravarthy, R., Naik, G. N., Savalia, A., Sridharan, U., Saravanan, C., Das, A. K., Gudasi, K. B. (2016). Determination of Naphthenic Acid Number in Petroleum Crude Oils and Their Fractions by Mid-Fourier Transform Infrared Spectroscopy. Energy & Fuels, 30 (10), 8579–8586. https://doi.org/10.1021/acs.energyfuels.6b01766
- Qu, D. R., Zheng, Y. G., Jing, H. M., Yao, Z. M., Ke, W. (2006). High temperature naphthenic acid corrosion and sulphidic corrosion of Q235 and 5Cr1/2Mo steels in synthetic refining media. Corrosion Science, 48 (8), 1960–1985. https://doi.org/10.1016/j.corsci.2005.08.016
- Moura, L. B., Guimarães, R. F., Abreu, H. F. G. de, Miranda, H. C. de, Tavares, S. S. M. (2012). Naphthenic corrosion resistance, mechanical properties and microstructure evolution of experimental Cr-Mo steels with high Mo content. Materials Research, 15 (2), 277–284. https://doi.org/10.1590/s1516-14392012005000024
- Huang, B. S., Li, H., Liu, Q. Y., Ma, X., Wang, Y. (2009). Inhibitor of naphthenic acid corrosion in atmospheric and vacuum distillation unit. Corrosion & Protection, 30 (10), 721–723. Available at: https://www.mat-test.com/en/article/id/5fa03a84-16a8-4975-9869-9e40a292caff
- Sun, M., Nicosia, D., Prins, R. (2003). The effects of fluorine, phosphate and chelating agents on hydrotreating catalysts and catalysis. Catalysis Today, 86 (1-4), 173–189. https://doi.org/10.1016/s0920-5861(03)00410-3
- Salem, S. M., Abdelaleem, G. M., Elsayed, N. A., Saad, W. O. (2011). Improving the qality of petroleum crude oils by deasphalting. JES. Journal of Engineering Sciences, 39 (4), 885–896. https://doi.org/10.21608/jesaun.2011.127722
- Tang, C., Farhadian, A., Berisha, A., Deyab, M. A., Chen, J., Iravani, D. et al. (2022). Novel Biosurfactants for Effective Inhibition of Gas Hydrate Agglomeration and Corrosion in Offshore Oil and Gas Pipelines. ACS Sustainable Chemistry & Engineering, 11 (1), 353–367. https://doi.org/10.1021/acssuschemeng.2c05716
- Wang, Q., Wang, R., Zhang, Q., Zhao, C., Zhou, X., Zheng, H. et al. (2023). Application of Biomass Corrosion Inhibitors in Metal Corrosion Control: A Review. Molecules, 28 (6), 2832. https://doi.org/10.3390/molecules28062832
- Kaban, A. P. S., Ridhova, A., Priyotomo, G., Elya, B., Maksum, A., Sadeli, Y. et al. (2021). Development of white tea extract as green corrosion inhibitor in mild steel under 1 M hydrochloric acid solution. Eastern-European Journal of Enterprise Technologies, 2 (6 (110)), 6–20. https://doi.org/10.15587/1729-4061.2021.224435
- Riastuti, R., Mashanafie, G., Rizkia, V., Maksum, A., Prifiharni, S., Kaban, A. et al. (2022). Effect of syzygium cumini leaf extract as a green corrosion inhibitor on API 5l carbon steel in 1M HCL. Eastern-European Journal of Enterprise Technologies, 6 (6 (120)), 30–41. https://doi.org/10.15587/1729-4061.2022.267232
- Muliarta, I. N., Darmawan, I. K. (2021). Processing Household Organic Waste into Eco-Enzyme as an Effort to Realize Zero Waste. Agriwar Journal, 1 (1), 6–11.
- Moradi, M., Topchiy, E., Lehmann, T. E., Alvarado, V. (2013). Impact of ionic strength on partitioning of naphthenic acids in water–crude oil systems – Determination through high-field NMR spectroscopy. Fuel, 112, 236–248. https://doi.org/10.1016/j.fuel.2013.05.024
- ASTM G5-94(1999)e1. Standard Reference Test Method for Making Potentiostatic and Potentiodynamic Anodic Polarization Measurements. https://doi.org/10.1520/g0005-94r99e01
- Soedarsono, J. W., Shihab, M. N., Azmi, M. F., Maksum, A. (2018). Study of curcuma xanthorrhiza extract as green inhibitor for API 5L X42 steel in 1M HCl solution. IOP Conference Series: Earth and Environmental Science, 105, 012060. https://doi.org/10.1088/1755-1315/105/1/012060
- Jayakumar, S., Nandakumar, T., Vadivel, M., Thinaharan, C., George, R. P., Philip, J. (2019). Corrosion inhibition of mild steel in 1 M HCl usingTamarindus indicaextract: electrochemical, surface and spectroscopic studies. Journal of Adhesion Science and Technology, 34 (7), 713–743. https://doi.org/10.1080/01694243.2019.1681156
- Vorobyova, V., Skiba, M. (2024). Mechanism of inhibitory action of fruit cake extracts as a new environmentally inhibitors of carbon steel corrosion. Results in Chemistry, 7, 101317. https://doi.org/10.1016/j.rechem.2024.101317
- Li, Y., Xu, W., Lai, J., Qiang, S. (2022). Inhibition Effect and Mechanism Explanation of Perilla Seed Extract as a Green Corrosion Inhibitor on Q235 Carbon Steel. Materials, 15 (15), 5394. https://doi.org/10.3390/ma15155394
- Liao, B., Ma, S., Zhang, S., Li, X., Quan, R., Wan, S., Guo, X. (2023). Fructus cannabis protein extract powder as a green and high effective corrosion inhibitor for Q235 carbon steel in 1 M HCl solution. International Journal of Biological Macromolecules, 239, 124358. https://doi.org/10.1016/j.ijbiomac.2023.124358
- Arslanhan, S., Sığırcık, G., Yıldız, R., Baran, M. F. (2024). Lavandula angustifolia Extract as a Green Corrosion Inhibitor for Protection of Mild Steel in HCl Acid Solution. Protection of Metals and Physical Chemistry of Surfaces, 60 (3), 554–570. https://doi.org/10.1134/s2070205124701739
- Banda-Cruz, E. E., Gallardo-Rivas, N. V., Martínez-Orozco, R. D., Páramo-García, U., Mendoza-Martínez, A. M. (2021). Derivative UV-Vis Spectroscopy of Crude Oil and Asphaltene Solutions for Composition Determination. Journal of Applied Spectroscopy, 87 (6), 1157–1162. https://doi.org/10.1007/s10812-021-01124-4
- Lin, T.-C., Cole, J. M., Higginbotham, A. P., Edwards, A. J., Piltz, R. O., Pérez-Moreno, J. et al. (2013). Molecular Origins of the High-Performance Nonlinear Optical Susceptibility in a Phenolic Polyene Chromophore: Electron Density Distributions, Hydrogen Bonding, and ab Initio Calculations. The Journal of Physical Chemistry C, 117 (18), 9416–9430. https://doi.org/10.1021/jp400648q
- Kochowski, S., Nitsch, K. (2002). Description of the frequency behaviour of metal–SiO2–GaAs structure characteristics by electrical equivalent circuit with constant phase element. Thin Solid Films, 415 (1-2), 133–137. https://doi.org/10.1016/s0040-6090(02)00506-0
- Elaraby, A., El-samad, Shrouk. A., khamis, Eman. A., Zaki, E. G. (2023). Theoretical and electrochemical evaluation of tetra-cationic surfactant as corrosion inhibitor for carbon steel in 1 M HCl. Scientific Reports, 13 (1). https://doi.org/10.1038/s41598-023-27513-7
- Vashishth, P., Bairagi, H., Narang, R., Shukla, S. K., Mangla, B. (2022). Thermodynamic and electrochemical investigation of inhibition efficiency of green corrosion inhibitor and its comparison with synthetic dyes on MS in acidic medium. Journal of Molecular Liquids, 365, 120042. https://doi.org/10.1016/j.molliq.2022.120042
- Sedik, A., Lerari, D., Salci, A., Athmani, S., Bachari, K., Gecibesler, İ. H., Solmaz, R. (2020). Dardagan Fruit extract as eco-friendly corrosion inhibitor for mild steel in 1 M HCl: Electrochemical and surface morphological studies. Journal of the Taiwan Institute of Chemical Engineers, 107, 189–200. https://doi.org/10.1016/j.jtice.2019.12.006
- Li, X.-H., Deng, S.-D., Fu, H. (2010). Inhibition by Jasminum nudiflorum Lindl. leaves extract of the corrosion of cold rolled steel in hydrochloric acid solution. Journal of Applied Electrochemistry, 40 (9), 1641–1649. https://doi.org/10.1007/s10800-010-0151-5
- Kaban, A. P. S., Soedarsono, J. W., Mayangsari, W., Anwar, M. S., Maksum, A., Ridhova, A., Riastuti, R. (2023). Insight on Corrosion Prevention of C1018 in 1.0 M Hydrochloric Acid Using Liquid Smoke of Rice Husk Ash: Electrochemical, Surface Analysis, and Deep Learning Studies. Coatings, 13 (1), 136. https://doi.org/10.3390/coatings13010136
- Xhanari, K., Finšgar, M., Knez Hrnčič, M., Maver, U., Knez, Ž., Seiti, B. (2017). Green corrosion inhibitors for aluminium and its alloys: a review. RSC Advances, 7 (44), 27299–27330. https://doi.org/10.1039/c7ra03944a
- Al Otaibi, N., Hammud, H. H. (2021). Corrosion Inhibition Using Harmal Leaf Extract as an Eco-Friendly Corrosion Inhibitor. Molecules, 26 (22), 7024. https://doi.org/10.3390/molecules26227024
- Huang, J., Hu, J., Cai, J., Huang, H., Wei, J., Yu, Q. (2022). Inhibition Effect of Hydrophobic Functional Organic Corrosion Inhibitor in Reinforced Concrete. Materials, 15 (20), 7124. https://doi.org/10.3390/ma15207124
- Raja, P. B., Qureshi, A. K., Abdul Rahim, A., Osman, H., Awang, K. (2013). Neolamarckia cadamba alkaloids as eco-friendly corrosion inhibitors for mild steel in 1M HCl media. Corrosion Science, 69, 292–301. https://doi.org/10.1016/j.corsci.2012.11.042
- Zou, Z., Liu, Z., Yang, L., Tang, Y., Qiao, Y., Lu, D. (2024). Corrosion behavior of different building planes of selective laser melting 316L stainless steel in 0.1 M HCl solution. Journal of Materials Research and Technology, 28, 4738–4753. https://doi.org/10.1016/j.jmrt.2024.01.078
- Yadav, A. K., Pandey, R., Singh, P. (2025). Impedance spectroscopy in perovskite materials: From fundamentals to applications. Inorganic Chemistry Communications, 182, 115524. https://doi.org/10.1016/j.inoche.2025.115524
- Meng, Y., Li, S., Zhang, Z. (2024). Inhibition performance of uniconazole on steel corrosion in simulated concrete pore solution: An eco-friendly way for steel protection. Heliyon, 10 (3), e24688. https://doi.org/10.1016/j.heliyon.2024.e24688
- Tang, Z., Huang, W., Liu, L., Li, H., Meng, H., Zeng, T. et al. (2024). Study on structure and molecular scale protection mechanism of green Ce,N-CDs anti-bacterial and anti-corrosive inhibitor. Journal of Materials Research and Technology, 28, 3865–3881. https://doi.org/10.1016/j.jmrt.2023.12.250
- Mabrouk, D. H., El-Morsy, F. E., Alsam, A. A. (2024). Electrochemical studies, adsorption behavior, and spectroscopic analysis of vanadyl complex of bis(1-(pyridin-2-yl)ethylidene)malonohydrazide as efficient eco-friendly corrosion inhibitor for low carbon steel in 1M HCl. International Journal of Electrochemical Science, 19 (5), 100528. https://doi.org/10.1016/j.ijoes.2024.100528
- Silva, R. M. P., Suffredini, H. B., Bastos, I. N., Santos, L. F., Simões, A. M. P. (2022). Naphthenic acid corrosion of API 5L X70 steel in aqueous/oil environment using electrochemical surface-resolved and analytical techniques. Electrochimica Acta, 407, 139900. https://doi.org/10.1016/j.electacta.2022.139900
- Pessu, F., Barker, R., Chang, F., Chen, T., Neville, A. (2021). Iron sulphide formation and interaction with corrosion inhibitor in H2S-containing environments. Journal of Petroleum Science and Engineering, 207, 109152. https://doi.org/10.1016/j.petrol.2021.109152
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2025 Kadek Ambara Jaya, Johny Soedarsono, Yudha Pratesa, Rini Riastuti, Agus Kaban

This work is licensed under a Creative Commons Attribution 4.0 International License.
The consolidation and conditions for the transfer of copyright (identification of authorship) is carried out in the License Agreement. In particular, the authors reserve the right to the authorship of their manuscript and transfer the first publication of this work to the journal under the terms of the Creative Commons CC BY license. At the same time, they have the right to conclude on their own additional agreements concerning the non-exclusive distribution of the work in the form in which it was published by this journal, but provided that the link to the first publication of the article in this journal is preserved.
A license agreement is a document in which the author warrants that he/she owns all copyright for the work (manuscript, article, etc.).
The authors, signing the License Agreement with TECHNOLOGY CENTER PC, have all rights to the further use of their work, provided that they link to our edition in which the work was published.
According to the terms of the License Agreement, the Publisher TECHNOLOGY CENTER PC does not take away your copyrights and receives permission from the authors to use and dissemination of the publication through the world's scientific resources (own electronic resources, scientometric databases, repositories, libraries, etc.).
In the absence of a signed License Agreement or in the absence of this agreement of identifiers allowing to identify the identity of the author, the editors have no right to work with the manuscript.
It is important to remember that there is another type of agreement between authors and publishers – when copyright is transferred from the authors to the publisher. In this case, the authors lose ownership of their work and may not use it in any way.









